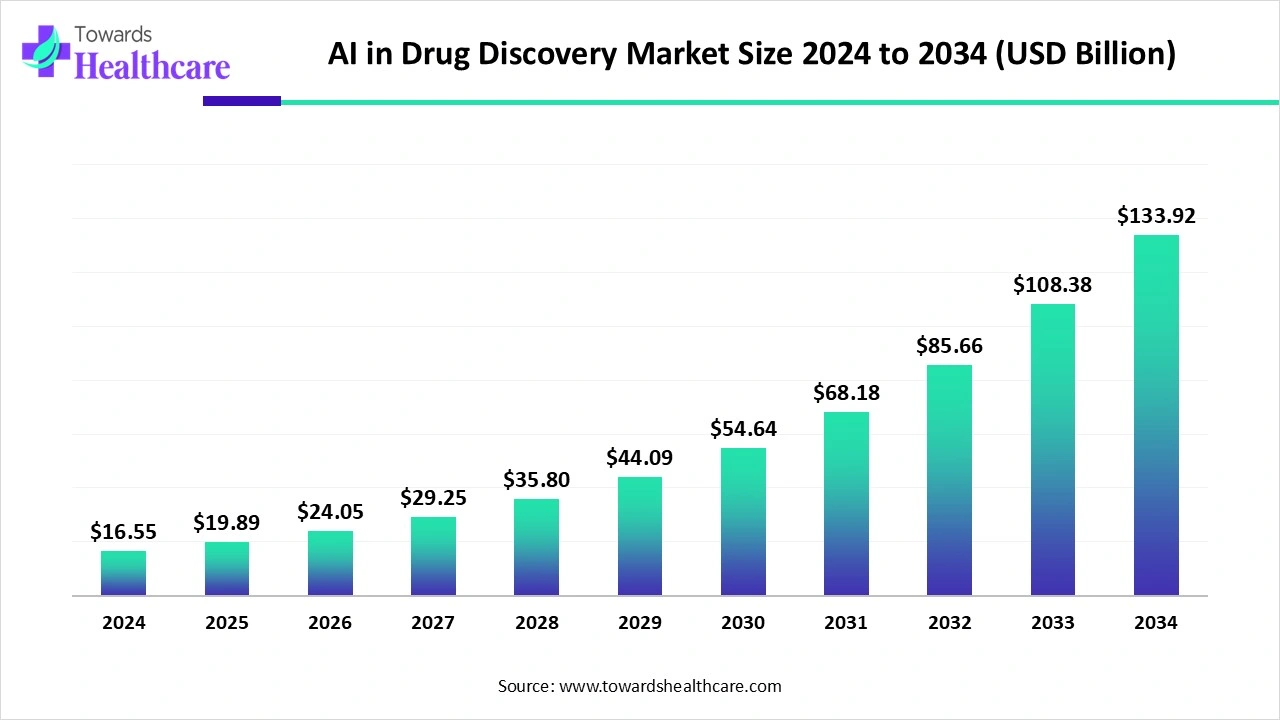
The global AI in Drug Discovery Market was valued at USD 19.89 billion in 2025 and is projected to grow to USD 133.92 billion by 2034 (CAGR 23.22% from 2024–2034), driven by AI’s ability to shorten R&D cycles, reduce costs, improve target identification and enable precision/repurposing strategies.
Download the free sample and get the complete insights and forecasts report on this market @ https://www.towardshealthcare.com/download-sample/5010
Market size
2025 baseline and long-term projection
◉Market size: USD 19.89 billion (2025).
◉Forecast: USD 133.92 billion (2034) — implies aggressive expansion as AI penetrates discovery and preclinical/clinical workflows.
Compound Annual Growth Rate (CAGR)
◉Stated CAGR: 23.22% (2024–2034) — consistent with rapid adoption, high venture funding and integration with cloud/computational infrastructure.
Regional contribution (snapshot & growth pattern)
◉North America dominates the absolute market (North America USD 11.32B in 2025, rising to USD 74.81B in 2034), indicating large incumbents, deep R&D budgets and cloud/AI infrastructure.
◉Europe and Asia Pacific show substantial growth trajectories (Europe USD 4.38B in 2025 → USD 27.98B in 2034; Asia Pacific ~USD 2.82B in 2025 → USD 22.38B in 2034), reflecting research ecosystems and expanding startups.
Market scaling dynamics
◉Absolute increase from 2025→2034: USD 114.03B, meaning large new addressable spend across pharma, biotech, CROs, and tools/platform providers.
◉Rapid top-line expansion indicates movement from pilot projects to productionized AI-driven discovery pipelines.
Unit economics & downstream value capture
◉AI vendors that can demonstrably improve hit-to-lead conversion, shorten timelines or reduce experimental cycles will capture disproportionate value (platforms, SaaS, compute & data services).
Investment and M&A signal
◉Multiple acquisitions and high funding rounds (e.g., startups raising >$1B or multiple acqui-hires) indicate investor conviction and consolidation potential.
Segment concentration (by solution type)
◉Core revenue pools: platform subscriptions (SaaS), project collaborations (co-discovery deals), licensing of AI-designed assets, and compute/data services.
Cost offsets and ROI
◉Given average cost to bring a drug to market (USD 2.6B), even modest reductions in attrition or time-to-candidate justify large vendor valuations and market spend.
Temporal adoption curve
◉Early adopters: large pharma and digitally mature biotech. Fast followers: CROs, niche biotech, academic translational units. Broad market: majority of mid-size pharma by late decade.
Sensitivity factors
◉Market size dependent on: data availability & sharing, regulatory comfort with AI outputs, demonstrated clinical validation of AI-derived molecules, and cloud/computing cost trends.
Market trends
Surging venture funding & unicorn/large rounds
◉Example: Xaira Therapeutics (Apr 2024) debut backed by >$1B funding — signals large capital inflows into platform biology + generative approaches.
Startups emerging in regional hotspots
◉India example: Peptris (Dec 2023) $1M pre-seed in Bangalore — shows geographic spread of innovation beyond traditional hubs.
Platform commercialization (SaaS + compute + generative AI)
◉MilliporeSigma’s AIDDISON™ (Dec 2023) — SaaS platforms that combine generative AI, ML and CADD (computer-aided drug design) are moving into commercial offerings.
Consolidation and capability build via M&A
◉Ginkgo Bioworks acquisitions (Feb 2024) to augment AI models, assays and RNA toolkits — trend toward vertical integration of wet lab + ML stacks.
Cloud & hyperscaler partnerships
◉Exscientia–AWS extension (July 2024) — demonstrates reliance on cloud ML/compute scale and the importance of partnerships with hyperscalers.
Regulatory & public policy moves shaping adoption
◉Examples: Canada creating a Canadian Drug Agency and funding rare disease strategy — government programs encourage faster translation and can drive demand for AI-enabled discovery.
Open model / community efforts
◉Google’s announcement of TxGemma (March 2025) — open AI models targeted at therapeutic entities accelerates researcher access and could democratize some capabilities.
Cross-industry collaborations
◉Pharma ↔ AI vendor collaborations (e.g., WeComput partnership) indicate co-development rather than pure vendor/customer relationships.
Shift from individual modules to end-to-end platforms
◉Movement from isolated tools (docking, QSAR, image analysis) to integrated pipelines covering target ID → optimization → preclinical prediction.
Expanding therapeutic focus
◉While oncology is currently largest application, infectious disease and polypharmacology research are gaining traction, fueled by pandemic lessons and vaccine/therapeutic R&D needs.
AI impacts / roles in drug discovery
1. Target identification & nomination (systematic hypothesis generation)
◉AI ingests multi-omic datasets, literature, clinical records and screens for correlated disease drivers.
Impact: reveals non-obvious targets (e.g., network hubs), reduces false leads, and ranks targets by druggability and safety risk.
2. Virtual screening & hit identification at scale
◉Deep models predict binding probability across enormous chemical spaces far faster than docking.
◉Impact: transforms screening from experimental high-throughput to virtual first pass, lowering reagent/time costs and expanding chemical diversity.
3. De novo molecular design and optimization
◉Generative models propose novel scaffolds optimized for potency, ADME properties, and synthesizability.
◉Impact: accelerates hit-to-lead cycles by offering candidate structures that balance multiple constraints simultaneously.
4. Predictive ADMET & toxicity modeling
◉AI predicts absorption, metabolism, off-target interactions and toxicity endpoints from structure + biology.
◉Impact: earlier elimination of unsafe chemotypes, reducing late-stage failures and costly animal studies.
5. Lead prioritization & multi-objective optimization
◉Multi-objective optimization algos balance potency, selectivity, PK, synthesis routes and cost.
◉Impact: reduces iterative wet-lab cycles required to converge on viable candidates.
6. Biomarker discovery & patient stratification for trials
◉ML on clinical data + omics finds biomarkers to select high-probability responder cohorts.
◉Impact: increases trial signal, decreases required sample size, and reduces trial duration and cost.
7. Clinical trial design optimization
◉AI models simulate trial outcomes, suggest adaptive designs, and propose enrollment criteria to minimize attrition.
◉Impact: improves probability of success; aligns with stated market advantage of reducing clinical cycle time and increasing productivity.
8. Drug repurposing and polypharmacology prediction
◉Network models and similarity embeddings uncover new indications for existing molecules and predict multi-target activity.
◉Impact: faster route to clinic (known safety profile), cost reduction, and addressing multifactorial diseases.
9. Automation + closed-loop wet lab (lab automation integration)
◉Integration of ML with robotic platforms closes the loop: propose → synthesize → assay → retrain.
◉Impact: accelerates iteration cadence from months to days; enables efficient model retraining with real experimental data.
10. Knowledge synthesis & decision support (literature / IP mining)
◉NLP models extract relationships from literature, patents, clinicaltrials, enabling faster diligence and hypothesis formation.
◉Impact: reduces duplication, surfaces prior art / constraints, and helps teams decide where to invest experiments.
Regional insights
North America (dominant market & innovation epicenter)
Market size & trajectory
◉USD 11.32B (2025) → USD 74.81B (2034): largest absolute market and fastest absolute dollar growth.
Enablers
◉Large pharma R&D budgets, concentration of AI/ML talent, advanced cloud infrastructure, robust VC ecosystems.
Business models
◉Enterprise deals (co-discovery, licensing), large partnerships with hyperscalers, and platform SaaS adoption.
Regulatory & payer dynamics
◉FDA engagement and pharma regulatory sophistication allow earlier pilots to scale; reimbursement pressure drives cost-saving tech adoption.
Risks
◉High cost base, competition for talent, and expectations for near-term ROI from investors.
Europe
Market size & trajectory
◉USD 4.38B (2025) → USD 27.98B (2034).
Enablers
◉Strong academic centers, translational institutes, and patient data registries; emphasis on precision medicine and public funding.
Regulatory posture
◉Balanced approach: robust data protection and clinical validation requirements — slows some rollouts but increases quality of evidence.
Strengths & opportunities
◉Leadership in biology-driven AI startups (Insilico, Owkin, Iktos, Deep Genomics) and collaborations with hospitals/biobanks.
Asia Pacific (fastest regional growth rate)
Market size & trajectory
USD 2.82B (2025) → USD 22.38B (2034) — high percentage growth as ecosystems mature.
Enablers
◉Growing biotech startups, lower operating costs for wet labs, improving regulatory frameworks, and massive population data for epidemiology.
Countries of note
◉China: rapid clinical trial registration growth (2022→2023), active regulatory updates; India: vibrant AI/ML talent pool and emerging biotech startups.
Risks & constraints
◉Data sharing/legal differences, fragmented market, and IP / commercialization pathways variability.
Latin America & Middle East & Africa (emerging but strategic)
Market size & trajectory
◉Latin America: USD 0.97B (2025) → USD 6.77B (2034).
◉MEA: USD 0.39B (2025) → USD 1.98B (2034).
Enablers
◉Localized research centers, increasing partnerships with global pharma, and interest in regional disease burdens.
Challenges
◉Infrastructure, funding, and limited local venture scale — adoption likely via partnerships and off-site cloud services.
Market dynamics
Demand drivers
◉Rising chronic disease burden (e.g., CDC stats referenced): more need for rapid, cost-effective therapeutics.
◉Economic pressure: average drug development cost (~USD 2.6B) incentivizes AI for cost reduction.
Supply dynamics
◉Growth of AI vendors offering diverse offerings (generative, predictive, platform, data curation).
◉Hyperscaler capacity and cloud pricing shape compute cost economics.
Competition & differentiation
◉Differentiators: quality/volume of training data, algorithmic novelty, wet-lab integration, regulatory evidence of clinical impact, and domain expertise.
Partnerships & business models
◉Typical models: SaaS subscriptions, milestone-based discovery partnerships, licensing, equity/joint ventures and acquisitions.
Regulatory & ethical constraints
◉Need for explainability, validation of AI predictions in vitro/in vivo and compliance with data privacy laws — affect time-to-market for platforms’ outputs.
Adoption barriers
◉Data access costs, skill gaps in pharma teams, concerns over model interpretability, and cultural inertia in lab practices.
Enablers of scale
◉Demonstrated case studies where AI outputs became clinically validated assets; public programs and funding (e.g., national drug strategies) can accelerate adoption.
Risk & mitigation
◉Risk: over-promising (hype) → mitigation: rigorous validation, transparent benchmarks, and phased pilots tied to experimental readouts.
Value capture
◉Vendors providing integrated wet-lab + model feedback loops or unique datasets can command premium pricing and strategic partnerships.
Outlook
◉Shift from exploratory pilots (2020s) to mainstream, regulated workflows by end of decade if AI consistently reduces attrition and accelerates candidate progression.
Top 10 companies
1. Exscientia
Product/Offering: End-to-end AI discovery and automation platform (design → optimization → candidate selection).
Overview: Publicly notable AI-drug discovery company; extended partnerships with AWS to scale ML and automation.
Strength: Integrated platform approach, strong industry partnerships, and demonstrated collaborations with pharma.
2. Insilico Medicine
Product/Offering: Generative chemistry and target ID platforms combining deep learning with biological datasets.
Overview: AI company focused on generative models to propose novel compounds and biomarkers.
Strength: Deep learning expertise applied to de novo design and translational biomarker discovery.
3. Atomwise
Product/Offering: Structure-based virtual screening and lead discovery using deep learning (atom-level models).
Overview: Early mover in ML docking/virtual screening.
Strength: Scalable virtual screening, sizable compound libraries, and pharma collaboration network.
4. IBM (Watson Health)
Product/Offering: ML/NLP platforms for biomedical data analysis, literature mining and decision support.
Overview: Large enterprise provider with cross-industry reach.
Strength: Enterprise credibility, large datasets, and integration capabilities for pharma customers.
5. Google (DeepMind / TxGemma)
Product/Offering: Advanced AI models (e.g., TxGemma — models understanding therapeutic entities, molecules, proteins).
Overview: Hyperscaler with leading research teams producing open/model toolkits for researchers.
Strength: World-class foundational models, compute resources, and potential to democratize access (open models).
6. BenevolentAI
Product/Offering: AI platform for target discovery, biomedical knowledge graphs and drug repurposing.
Overview: Knowledge graph + ML approach to accelerate hypothesis generation.
Strength: Graph-based knowledge synthesis and translational focus.
7. Aitia (formerly GNS Healthcare)
Product/Offering: Causal ML and simulation models for patient stratification and drug response prediction.
Overview: Rebranded/former GNS Healthcare — expertise in translating complex data to clinical predictions.
Strength: Causal inference focus and real-world evidence modeling strengths.
8. BioSymetrics, Inc.
Product/Offering: ML platforms for biomarker discovery and patient stratification tied to drug discovery pipelines.
Overview: Specializes in combining clinical and molecular datasets for translational insights.
Strength: Deep domain expertise in biomarkers and clinical genomics.
9. Insitro
Product/Offering: Machine learning + high-throughput biology to generate disease models and discover targets.
Overview: Uses data generation (cellular/tissue assays) integrated with ML to produce validated insights.
Strength: Integration of wet-lab data generation with ML model training loops.
10. Berg Health (now part of BPGbio Inc.) / CYCLICA (acquired by Recursion) — combined note
Product/Offering: Berg: biologically driven AI platforms; CYCLICA: cyclic peptide discovery tech (now within Recursion).
Overview: Acquisitions indicate consolidation and absorption of niche technologies into larger AI discovery firms.
Strength: Domain-specific IP (e.g., cyclic peptides) and biologically grounded discovery approaches.
Latest announcements
Google — TxGemma (March 2025)
What: Announcement of TxGemma, an open collection of AI models that understand therapeutic entities (small molecules, proteins, chemicals).
Implication: Democratizes access to specialized models for researchers; accelerates hypothesis testing and property prediction across modalities.
Ginkgo Bioworks acquisitions (Feb 2024)
What: Acquired Reverie Labs, Proof Diagnostics, Patch Biosciences.
Implication: Strengthens Ginkgo’s AI foundation models, RNA tools and ML-assay capabilities — clarifies trend of combining biological hardware with ML stacks.
MilliporeSigma — AIDDISON™ (Dec 2023)
What: SaaS platform combining generative AI, ML and CADD to boost success rates of therapies.
Implication: Large legacy life-science companies pivoting to provide platformized AI drug discovery tools.
Exscientia–AWS extension (July 2024)
What: Extended partnership to leverage AWS ML and AI capabilities for Exscientia’s automation platform.
Implication: Shows critical role of hyperscalers in providing scalable compute and ML infrastructure for AI drug discovery operations.
Iambic Therapeutics (Oct 2024)
What: Claimed release of an AI model that could significantly reduce time and cost to develop new drugs.
Implication: Early stage claims of transformative models continue to surface; emphasizes need for independent validation.
Accenture investment in 1910 Genetics (Oct 2024)
What: Accenture Ventures investment into 1910 Genetics to advance multimodal AI + lab automation for small/large molecules.
Implication: Consultancies and systems integrators are placing strategic bets on multimodal, automated discovery platforms.
Recent developments
Massive funding events & new startups
Xaira Therapeutics (Apr 2024) debut with >$1B financing illustrating large capital flows into platform drug discovery.
Startups raising seed/pre-seed globally
Peptris Technologies (Dec 2023) in Bangalore — shows expansion of AI drug discovery ecosystems outside US/Europe.
Hyperscaler & pharma partnerships
Exscientia + AWS (Jul 2024) and similar collaborations embed cloud ML capabilities into discovery pipelines.
Consolidation via M&A
Ginkgo Bioworks acquisitions (Feb 2024) and CYCLICA acquisition (May 2023 by Recursion) — vertical consolidation of wet-lab, assay and AI capabilities.
Enterprise product launches
MilliporeSigma’s AIDDISON™ (Dec 2023) — enterprise SaaS offerings from established life-science vendors.
Open model efforts
Google’s TxGemma (Mar 2025) — trend to release therapeutic-aware foundational models.
Government & policy moves
Canada’s National Strategy for Drugs for Rare Diseases and CDA formation — public funding and agency activity to support drug pipelines where AI can help.
Clinical trial & regulatory environment changes
China NMPA seeking input to expedite foreign innovative drugs (June 2024) — indicates regulatory modernization that may help AI-discovered assets enter markets.
Academic & industry cross-pollination
Owkin and pathologist workflow initiatives (Jan 2025) — digital pathology + AI to relieve clinician burden and feed discovery pipelines.
Claims of transformational AI models
Iambic (Oct 2024) and others announcing models that purport to reduce time/cost — signal both innovation and the need for independent validation.
Segments covered
1. Solution type / technology
Subpoints: Generative models, deep learning, supervised ML, reinforcement learning, knowledge graphs, CADD integrations.
Explanation: Each technology targets specific discovery tasks — generative models for design, deep learning for pattern recognition in imaging/omics, knowledge graphs for hypothesis linking.
2. Platform vs Project services
Subpoints: SaaS platforms (subscription), project-based collaborations (milestone payments), licensing.
Explanation: Platforms scale across customers; projects are bespoke and often the route for high-value co-discovery deals.
3. Data & compute services
Subpoints: Curated datasets, private/pseudonymized clinical data, compute credits (hyperscalers).
Explanation: Data is a competitive moat; compute enables model training and inference at scale.
4. Wet-lab integration / automation
Subpoints: Robotic synthesis, high-throughput screening integration, closed-loop experimentation.
Explanation: Essential for closing ML loops and accelerating empirical validation of AI hypotheses.
5. Validation & regulatory evidence generation
Subpoints: In vitro, in vivo, translational biomarkers, RWE (real-world evidence).
Explanation: Demonstrable experimental validation builds credibility, necessary for licensing and regulatory acceptance.
6. Specialty modalities
Subpoints: Small molecules, biologics, RNA modalities, cyclic peptides.
Explanation: Different modalities require tailored ML models and wet lab capabilities.
7. Clinical enablement & trial optimization
Subpoints: Biomarkers, patient selection, adaptive trial simulations, endpoint prediction.
Explanation: Downstream value in improving trial success probability and speed.
8. Repurposing & polypharmacology platforms
Subpoints: Knowledge graph repurposing, network pharmacology.
Explanation: Fastest route to clinic when safety profiles are known — high ROI for proven signals.
9. IP, legal & commercialization support
Subpoints: Patent landscaping, freedom-to-operate screening via NLP.
Explanation: Important to convert AI discoveries into protectable commercial assets.
10. Professional services & consultancy
Subpoints: Integration, validation, regulatory strategy services.
Explanation: Critical for enterprise customers lacking in-house AI/drug discovery expertise.
Top 5 FAQs
Q1: What is the current market size and projected growth for AI in drug discovery?
A: The market was evaluated at USD 19.89 billion in 2025 and is expected to reach USD 133.92 billion by 2034, growing at a 23.22% CAGR (2024–2034).
Q2: Which region currently leads the AI in drug discovery market?
A: North America leads in absolute terms (USD 11.32B in 2025, growing to USD 74.81B in 2034) due to large pharma presence, funding, cloud infrastructure and talent.
Q3: How does AI actually reduce drug discovery costs and timelines?
A: AI accelerates target ID, virtual screening, de novo design, ADMET prediction and trial design, which reduces wet-lab cycles, lowers attrition and compresses timelines — valuable given the typical drug development cost of USD 2.6 billion and low post-Phase I approval rates (<10%).
Q4: Who are major players to watch?
A: Key companies include Exscientia, Insilico Medicine, Atomwise, IBM (Watson), Google/DeepMind (TxGemma), BenevolentAI, Aitia (GNS), BioSymetrics, Insitro, and firms absorbed by larger players such as CYCLICA/Recursion.
Q5: What are the main barriers to broader adoption?
A: Primary restraints include data access/costs, model explainability, regulatory validation needs, high technical/infrastructure costs, and the cultural/organizational shift required in pharma R&D.
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5010
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Powering Healthcare Leaders with Real-Time Insights: https://www.towardshealthcare.com/healthcare-intelligence-platform
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest