The CAR T-cell neurotoxicity detection market is witnessing remarkable growth as the adoption of CAR T-cell therapies accelerates worldwide. These therapies, known for their promise in treating hematologic malignancies, have also highlighted the urgent need for early neurotoxicity detection due to risks like ICANS (immune effector cell-associated neurotoxicity syndrome). This article explores key trends, drivers, challenges, and opportunities shaping this market in 2025.
What is CAR T-Cell Neurotoxicity Detection?
CAR T-cell neurotoxicity detection involves tools, technologies, and services designed to identify and monitor neurotoxic side effects in patients receiving CAR T-cell therapy. Since neurotoxicity can range from mild symptoms like headaches to life-threatening complications, early detection through biomarkers, imaging, EEG, and AI-driven tools is critical for improving patient outcomes.
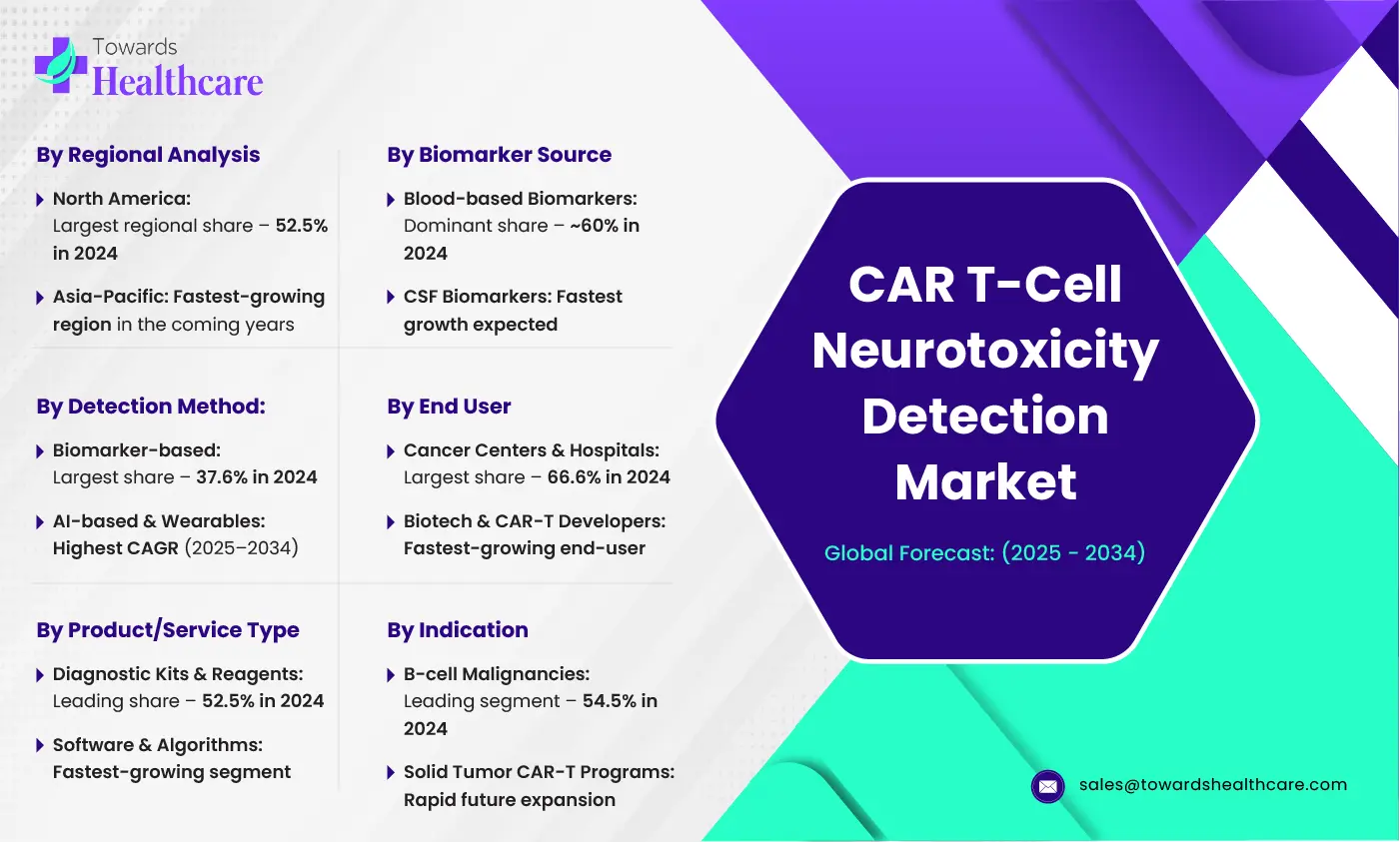
Key Market Insights 2025
Regional Insights
-
North America led the global market with a 52.5% revenue share in 2024, thanks to strong R&D infrastructure and regulatory support.
-
Asia-Pacific is projected to grow at the fastest rate, fueled by a surge in medtech startups and healthcare investments.
Segment Insights
-
By Detection Method: Biomarker-based detection dominated with a 37.6% market share, while AI-based & wearable monitoring is set to grow at the highest CAGR from 2025 to 2034.
-
By Product/Service: Diagnostic kits & reagents captured 52.5% of the market, but software & algorithms will experience the fastest growth.
-
By Biomarker Source: Blood-based biomarkers held 60% of the share due to ease of testing.
-
By End User: Cancer centers & hospitals accounted for 66.6% of revenue in 2024.
Invest in Our Premium Strategic Solution: https://www.towardshealthcare.com/download-databook/5802
Market Growth Drivers
Rising Prevalence of Blood Cancers
With around 187,740 people diagnosed globally in 2024, the demand for CAR T-cell therapy—and tools to monitor its effects—is soaring.
Advancements in AI and Genomics
AI enhances MRI and EEG diagnostics, providing rapid and precise detection of neurotoxic signs. Genomic technologies like CRISPR also pave the way for safer CAR T-cells.
Favorable Government Support
Governments are funding research, launching public awareness campaigns, and supporting innovative solutions to tackle neurotoxicity.
Challenges Hindering Market Expansion
The high cost of advanced diagnostic tools remains a major barrier, especially in low- and middle-income countries where affordability limits access.
Future Outlook: A Promising Decade Ahead
The future of the CAR T-cell neurotoxicity detection market looks bright. Researchers are exploring innovative biomarkers in cerebrospinal fluid and leveraging AI for predictive modeling. As of June 2025, five phase 4 clinical trials are investigating CAR T-cell therapies and their side effects.
Startups in China and India are also contributing significantly, focusing on early disease detection and AI-powered diagnostics.
Get All the Details in Our Solutions – Access Report Preview: https://www.towardshealthcare.com/download-sample/5802
Latest Industry Developments
-
March 2025: Kyushu University researchers identified proteins in cerebrospinal fluid linked to neurotoxicity, offering predictive insights for patient care.
-
June 2023: German and U.S. researchers developed a blood test measuring serum neurofilament light chain (NfL), aiding early detection of ICANS.
If you have any questions, please feel free to contact us at sales@towardshealthcare.com
Frequently Asked Questions (FAQs)
Q. What is CAR T-cell neurotoxicity, and why is detection important?
CAR T-cell neurotoxicity refers to harmful effects on the nervous system caused by CAR T-cell therapy. Early detection is vital to prevent severe complications like ICANS.
Q. How is AI changing the neurotoxicity detection landscape?
AI improves imaging accuracy, analyzes patient data in real time, and enables continuous monitoring with wearable devices, making detection faster and more precise.
Q. Which region leads the CAR T-cell neurotoxicity detection market in 2025?
North America holds the largest market share due to advanced healthcare infrastructure and regulatory initiatives supporting CAR T-cell therapies.
Q. Why are blood-based biomarkers so widely used?
Blood-based biomarkers are preferred because they provide a less invasive, faster, and cost-effective method for detecting neurotoxic effects.
Q. What challenges could slow market growth?
High costs of diagnostic technologies and limited access in developing regions are key hurdles to widespread adoption.
To access the full market report : https://www.towardshealthcare.com/price/5802