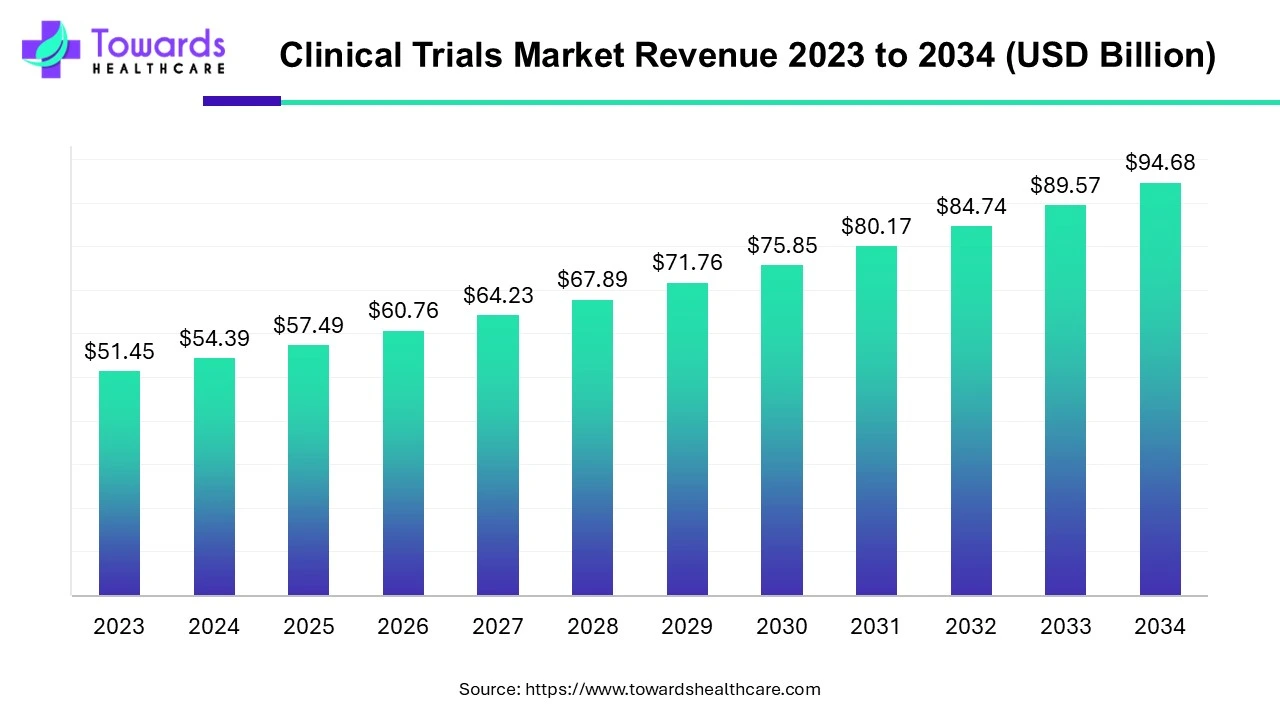
The global clinical trials market was USD 54.39 billion in 2024 and is projected to reach USD 94.68 billion by 2034 (CAGR 5.7% from 2024–2034), driven by rising R&D spend, personalized medicine, and rapid adoption of decentralized/virtual trials.
Download the free sample and get the complete insights and forecasts report on this market @ https://www.towardshealthcare.com/download-sample/5029
Market size
◉Current base (2024): USD 54.39B (user data).
◉Forecast (2034): USD 94.68B (user data) — implies cumulative growth reflecting expanded trial activity, outsourcing and digital/remote models.
◉Compound annual growth rate: 5.7% (2024–2034) — growth driven by higher trial volumes (oncology, rare diseases), more complex biologics, and regulatory acceleration programs.
◉Registries as a proxy for activity: ClinicalTrials.gov and similar registries hold hundreds of thousands of records (ClinicalTrials.gov > 400k registered studies historically), indicating large and growing global trial throughput.
◉Market composition by spend (high-level): sponsor R&D budgets (largest), CRO services, site payments, patient engagement & retention services, decentralized trial platforms, data management/analytics, and regulatory/compliance services. (Detailed dollar splits vary by year and sponsor strategy.)
◉CapEx & technology investment: major portion of incremental spend is on data platforms (EHR/RWE integration), remote monitoring, eConsent/telemedicine, AI patient-matching and analytics, and logistics for decentralized trial kits (invested both by sponsors and CROs).
Market trends
◉Decentralized & hybrid trials (accelerated by COVID-19): rapid shift to remote visits, home nursing, ePROs, and device/wearable monitoring which reduces site burden and broadens recruitment. (User examples: Northwell famotidine trial; ACTIV partnership.)
◉Regulatory modernization & harmonization: many regulators introduced faster pathways (e.g., FDA CTAP during COVID) and the EU implemented CTIS/CTR to centralize submissions (CTIS launched 31 Jan 2022; mandatory use for new applications by Jan 31, 2023). This reduces administrative friction for multisite EU trials.
◉Rising trial counts & registry transparency: ClinicalTrials.gov lists hundreds of thousands of trials across 200+ countries (registry expansion improves visibility and post-study reporting mandates).
◉Patient-centricity as core design principle: co-design of protocols with patients; minimizing visits; reimbursement/transport support; broader inclusion criteria to capture diverse populations.
◉Personalized medicine & biomarker-led trials: smaller, richer cohorts (basket/umbrella trials) that target molecularly defined subpopulations — increases complexity but improves probability of success for targeted therapies.
◉Real-World Evidence (RWE) & hybrid endpoints: sponsors increasingly combine RWE (EHRs, registries, wearables) with traditional outcomes to supplement efficacy/safety evidence.
◉AI & advanced analytics: used for site selection, patient matching, synthetic control arms, anomaly detection in safety data and adaptive designs — improving speed and lowering screen-failure rates.
◉Globalization with localized complexity: more trials in Asia Pacific, Latin America and Eastern Europe to access patient pools and lower costs — but each region requires localization: language, regulatory and ethics approvals, data privacy compliance.
◉Commercial & operational consolidation: large CROs expand capability sets (end-to-end services), while niche vendors focus on decentralized tools, eConsent, and analytics — leading to strategic partnerships and M&A.
◉Supply chain/logistics sophistication: home delivery of investigational products, cold-chain for biologics, and virtual site monitoring demand resilient logistics and qualified courier networks.
AI impact & role
1 Pre-trial planning & feasibility
◉AI-driven site selection: ML models ingest historical recruitment, site performance, investigator profiles and local prevalence to rank sites by projected enrollment speed and retention. This reduces start-up cycles and screen-failure rates.
◉Synthetic feasibility modeling: generative and predictive models simulate enrollment scenarios under different inclusion/exclusion criteria to optimize protocol design and timelines.
2 Patient identification & recruitment
◉EHR mining & phenotyping: NLP + structured data pipelines scan EHRs to identify eligible patients faster while preserving PHI through federated learning/secure enclaves.
◉Digital advertising optimization: AI optimizes ad targeting (social, search) to reach likely candidates and improves conversion metrics through continuous learning.
3 Protocol design & adaptive trials
◉In silico trial simulations: Bayesian/AI models simulate arms, dosing, and adaptive decision rules to refine sample sizes and stopping boundaries prior to first patient in.
◉Adaptive randomization & response-adaptive strategies: real-time analytics inform arm allocation to increase probability of success and patient benefit.
4 Operational monitoring & risk-based monitoring (RBM)
◉Anomaly detection in EDC: unsupervised and supervised models flag outliers, data fabrication patterns, and safety signal anomalies for targeted source data verification, reducing monitoring cost.
◉Predictive site risk scores: models forecast which sites may underperform or have data quality issues enabling pre-emptive remediation.
5 Safety surveillance & pharmacovigilance
◉Signal detection across heterogeneous data: AI aggregates trial data, spontaneous reporting, and RWE to prioritize adverse event signals and speed safety committee reviews.
◉Automated SAE coding & narrative generation: NLP automatically maps verbatim text to MedDRA, creating structured case reports for regulatory submissions.
6 Endpoint measurement & digital biomarkers
◉Sensor / wearable analytics: CV, movement, sleep, and activity metrics are processed by deep learning to create objective endpoints (e.g., gait stability, seizure detection).
◉Composite digital endpoints: AI fuses multimodal streams (video, audio, sensor, ePRO) into validated outcome variables.
7 Patient engagement & retention
◉Personalized nudges & chatbots: conversational AI delivers medication reminders, symptom checkers and tailored retention interventions, improving adherence and reducing dropout.
◉Sentiment & risk scoring: models analyze patient interactions to predict attrition risk and trigger clinical outreach.
8 Data curation & regulatory submission
◉Automated datasets & submission packages: AI pipelines assemble ADaM/SDTM-like datasets, generate analysis shells, and preflight checks for common regulatory deficiencies.
◉Document summarization & translation: NLP creates investigator brochures, translations and patient-facing materials more rapidly while preserving key facts.
9 Analysis, modeling & interpretation
◉Accelerated statistical modeling: Bayesian/ML hybrids accelerate subgroup discovery and covariate adjustments; enables more efficient use of smaller personalized cohorts.
◉Explainability & audit trails: growing requirement to add interpretability layers (SHAP, LIME) so regulators and auditors can review model rationale.
10 Governance, privacy & bias mitigation
◉Federated learning / privacy-preserving ML: allows model training across institutions without moving PHI.
◉Bias detection frameworks: systematic checks to ensure models don’t exclude underrepresented groups — critical for regulatory acceptance and ethics boards.
Regional Insights
1 North America (U.S. & Canada)
Dominant spend & sponsor concentration
◉The U.S. is home to the largest number of top 20 global pharmaceutical and biotech firms.
◉CRO headquarters concentration: Many of the top global CROs (IQVIA, Parexel, PPD) are U.S.-based, anchoring sponsor-CRO collaborations.
◉This creates a scale effect: sponsors can run high-budget, multi-indication programs with integrated preclinical-to-commercial solutions in one geography.
Regulatory acceleration & transparency
◉FDA initiatives: Breakthrough Therapy Designation, Accelerated Approval, and Oncology Center of Excellence programs have cut timelines significantly for high-need therapies.
◉COVID-19-era reforms (CTAP, EUA pathways) set precedents for rapid data review and rolling submissions.
◉Net effect: U.S. trials often start first globally, with early access to novel modalities (cell/gene therapies, mRNA vaccines, precision oncology drugs).
High technology adoption
◉Broad adoption of decentralized trial technologies: eSource, remote monitoring, RBM (Risk-Based Monitoring), AI-driven feasibility.
◉U.S. sites are typically first adopters of advanced data capture systems, creating faster learning cycles for new tech.
◉Academic medical centers (e.g., MD Anderson, Mayo, Dana-Farber) anchor early-phase and high-complexity trial ecosystems.
Patient advocacy & diverse recruitment pressure
◉Patient advocacy groups (esp. in oncology, rare diseases, HIV) lobby regulators and sponsors for inclusion and accelerated access.
◉FDA mandates diversity plans for pivotal trials; recruitment incentives increasingly tied to socio-economic outreach.
◉Challenge: Persistent underrepresentation of minority and rural patients despite technology-enabled outreach.
2 Europe (EU / UK)
Harmonization via CTR & CTIS
◉Clinical Trials Regulation (CTR, Jan 2022) + CTIS platform centralize submissions across EU/EEA.
◉Benefits: Faster multi-country start-up, standardized documentation, improved transparency.
◉UK (post-Brexit) is pursuing MHRA fast-track models to remain competitive.
High regulatory standards & data privacy
◉GDPR = strictest global regime on patient data. Sponsors must build privacy-aware RWE/registry pipelines.
◉Data localization laws (Germany, France) complicate multinational data flows, requiring hybrid data architecture.
Strong academic networks
◉Pan-European cooperative groups in oncology, cardiology, and rare disease trials (e.g., EORTC) attract global partnerships.
◉Sponsors increasingly outsource to CROs for managing complex biologics/advanced therapies in EU markets, where regulatory documentation and GMP are highly demanding.
3 Asia-Pacific (China, India, Japan, Southeast Asia)
Rapid growth & patient access
◉Large treatment-naïve patient pools (esp. oncology, infectious diseases, metabolic syndromes).
◉Cost arbitrage: per-patient trial costs 30–50% lower vs. U.S./EU.
◉Sponsors use APAC for Phase III global recruitment acceleration and local bridging studies.
Regulatory modernization
◉China (NMPA reforms, ICH alignment, faster IND review timelines).
◉India (new NDCT rules, ethics harmonization, trial insurance mandates).
◉Japan: still slower but investing in regenerative medicine fast-tracks.
◉These reforms boost data credibility, encouraging global sponsors to include APAC data in pivotal filings.
Localization & capacity building
◉Need for cultural adaptation in informed consent, language-specific patient materials.
◉Investment in investigator training and infrastructure (biobanks, cold chain) critical for trial quality.
4 Latin America
Faster recruitment & diversity
◉Patient willingness is high, especially in oncology, infectious diseases, and vaccines.
◉Adds valuable ethnic and genetic diversity to global datasets.
◉Sponsors leverage this region to rescue slow-recruiting U.S./EU trials.
Regulatory variability
◉Brazil (ANVISA) and Mexico show increasing alignment with ICH, but timelines still inconsistent.
◉Country-by-country submission strategies often required, slowing multi-country trial launches.
◉Site infrastructure improving, especially in urban research centers.
5 Middle East & Africa (MEA)
Emerging markets with untapped populations
◉GCC nations (UAE, Saudi Arabia, Qatar) investing in trial hubs (precision medicine, oncology).
◉Africa: Large untapped patient pools in infectious diseases and chronic illness — but infrastructure gaps persist.
Infrastructure & ethics capacity building
◉Need for harmonized ethics committees and GCP-compliant trial centers.
◉Logistics challenge: cold chain for biologics and cell therapies.
◉Sponsors often partner with local ministries of health to build capacity before launching large-scale trials.
Market Dynamics
1 Drivers
◉Rising R&D budgets & complex pipelines → biologics, cell & gene therapies require longer, more costly trials.
◉Personalized medicine & biomarkers → precision subgroups require adaptive and stratified trial designs.
◉Decentralization & digital health → broader patient reach, reduces geographic/logistical barriers.
◉Regulatory reforms & accelerated pathways → faster approvals motivate early-phase investment.
2 Restraints
◉Operational complexity & data heterogeneity → integrating EHR, wearables, lab data = non-trivial.
◉Regulatory & privacy barriers → GDPR, HIPAA, national data sovereignty laws.
◉Recruitment & retention challenges → rare disease cohorts, fragile elderly populations, pediatric cases.
◉Rising trial costs → biologics manufacturing, hybrid monitoring, and supply logistics inflate budgets.
3 Opportunities
◉AI & analytics platforms → predictive recruitment, automated data cleaning, adaptive trial optimization.
◉RWE & synthetic controls → reduce control-arm burden, accelerate submissions.
◉Niche CRO services → home nursing, decentralized orchestration, telehealth endpoints.
◉Emerging geographies → faster recruitment, cost efficiencies, underrepresented genetic populations.
4 Other Dynamics
◉Consolidation & partnerships → top CROs expanding into digital platforms and AI alliances.
◉Investor & VC interest → funding in digital trial platforms, decentralized models, AI-powered analytics remains robust.
Top Companies
1 IQVIA
◉Overview: Largest global CRO + healthcare data powerhouse.
◉Capabilities: End-to-end CRO services, AI site feasibility, RWE products (claims/EHR databases).
◉Strengths: Integrated clinical-commercial lifecycle, unmatched data depth for trial optimization.
2 Parexel
◉Overview: Leading CRO with strong regulatory & late-phase expertise.
◉Capabilities: Clinical development, eClinical tools, regulatory/market access advisory.
◉Strengths: Deep EMA/FDA regulatory know-how, broad site relationships, strong in biologics.
3 Charles River Laboratories
◉Overview: Preclinical leader expanding into translational/early clinical support.
◉Capabilities: Safety/toxicology, biologics testing, IND-enabling studies.
◉Strengths: Accelerates bench-to-IND readiness, complements CRO partners in later phases.
4 PPD (Thermo Fisher Scientific subsidiary)
◉Overview: Full-service CRO with lab and supply chain capabilities.
◉Capabilities: Clinical development, central labs, supply packaging/logistics.
◉Strengths: Scale, lab depth, and operational excellence across multiple therapeutic areas.
5 Omnicare / Kendle / Chiltern (historical niche players)
◉Overview: Now absorbed into larger entities, but historically strong in specialized therapeutic areas.
◉Strengths: Long-term site relationships, niche service lines that shaped CRO evolution.
Latest announcements & developments
1) Melvin & Bren Simon Cancer Center — REGN5459 (Apr 2023)
◉What happened (summary): first-in-human multiple myeloma trial showing ~90.5% overall response rate at higher doses (early cohorts).
Why it matters
◉Clinical validation speed: such high early ORR shortens the evidence gap between preclinical promise and clinical signal — accelerates dose expansion & pivotal planning.
◉Investor & partner interest: strong early signals draw capital, licensing conversations, and faster CRO/supply commitments.
◉Regulatory attention: early high responses may justify Breakthrough Designation / accelerated pathways (if safety profile acceptable).
Operational implications
◉Rapid scale-up needs: need to expand sites, logistics for increased IMP supply, and central lab throughput.
◉Biomarker & companion diagnostics: likely requirement to standardize assays across sites — QC and cross-lab harmonization essential.
◉Safety surveillance: intensive pharmacovigilance and DSMB cadence during expansion; SAE workflows must be prepped.
KPIs to monitor
◉time from dose-expansion decision to first patient enrolled (target: weeks–months),
◉IMP manufacturing lead time and lot release rate,
◉protocol amendment turnaround time (regulatory + site activation).
Risks & mitigations
◉Risk: early signal not durable → Mitigation: implement robust, pre-specified durability endpoints and interim analyses.
◉Risk: supply bottlenecks for biologic drug product → Mitigation: multiple manufacturing slots and safety stock.
2) MD Anderson at AACR 2023 — multiple early oncology signals
Why it matters
◉Confirms oncology continues to be the innovation engine, with multiple academic centers rapidly translating biology into trials.
◉Universities accelerate first-in-human work which then moves to industry-sponsored registrational programs.
Operational implications
◉Academic-to-industry handoffs: standardized data formats and early engagement with sponsor regulatory teams accelerate IND-to-CTA transitions.
◉Site capacity management: top academic centers become bottlenecks — expand investigator network planning early.
3) EU CTIS adoption (Jan 2022/2023) — centralized EU submissions
Why it matters
◉Single portal reduces duplication but increases transparency and harmonization across member states.
◉Mandatory use changed submission lifecycles and resource planning.
Operational implications
◉Regulatory submission redesign: templates, translations, and country-specific annexes need central coordination.
◉Public disclosure planning: sponsors must manage communication strategy for trial registries & posted documents.
Practical checklist
◉map CTIS dossier structure to internal CTMS,
◉pre-plan translations and local investigator brochures,
◉assign a CTIS submission owner and QPPV (where applicable).
4) COVID-19 legacy — decentralized trials, telemedicine, remote monitoring
Why it matters
◉DCTs moved from pilot to standard — reduced patient burden, improved reach, and changed monitoring models.
Operational implications
◉Hybrid protocol design: mix site visits with home nursing, eConsent, wearable endpoints.
◉Supply & logistics: home delivery of IMPs, kit returns, chain-of-custody for samples.
◉Monitoring model: RBM + central monitoring dashboards replace some SDV; on-site visits targeted.
Key performance changes (typical, illustrative)
◉enrollment speed ↑, screen-failures ↓, monitoring travel costs ↓ — measure these per trial phase to quantify ROI.
Risks & mitigations
◉Data integrity concerns from decentralized capture → implement eSource validation, timestamped audit trails, and device calibration SOPs.
5) Rising registered trials — ClinicalTrials.gov ~400k+ entries
Why it matters
◉Reflects growing trial volume, transparency, and competition for patient recruitment.
◉Public registry density increases ability to plan global feasibility but also elevates need for differentiation in recruitment outreach.
Operational implications
◉Feasibility complexity: more competing trials in the same indications → sponsors must target under-served regions or adopt DCTs.
◉Competitive intelligence: continuous registry scanning to identify overlapping trials and avoid recruitment cannibalization.
6) Biotech-driven pipeline — cell & gene therapies, cold-chain logistics
Why it matters
◉ATMPs and personalized biologics demand specialist CROs/suppliers and logistics (chain-of-custody, cryopreservation).
◉Drives demand for centralized manufacturing, regional hubs, and cell therapy couriers.
Operational implications
◉Extended supply chain planning: manufacturing slots, release testing, and regional distribution must be booked much earlier.
◉Regulatory & quality: sterility assurance, lot comparability and chain-of-identity documentation become critical.
KPIs
◉turnaround time for apheresis → product infusion, percentage of shipments meeting temperature range, lot-release time.
Segments Covered
1 By Phase — operational depth & resource profile
Phase I (First-in-Human)
◉Focus: safety, tolerability, PK/PD.
◉Resource needs: specialized early-phase units, intensive PK sampling, bioanalytics, GMP IMP supply at small scale.
◉Operational notes: rapid protocol amendments common; adaptive dose escalation (3+3, Bayesian model) and cohort expansion require nimble monitoring and rapid lab turnaround.
Phase II (Proof-of-Concept)
◉Focus: efficacy signals, dose optimization, biomarker validation.
◉Resource needs: central labs for biomarker assays, imaging core labs, statisticians for interim analyses.
◉Operational notes: adaptive designs reduce sample size but require sophisticated DSMB and statistical plan.
Phase III (Confirmatory)
◉Focus: definitive efficacy/safety, regulatory submission data.
◉Resource needs: global site network, logistics for large IMP volumes, full pharmacovigilance infrastructure, data lock and submission teams.
◉Cost drivers: number of sites, duration, complexity of endpoints (event-driven trials can extend timelines).
◉Operational notes: long lead times for site activation and country approvals—start early in project timelines.
Phase IV (Post-Marketing)
◉Focus: long-term safety, label expansion, RWE.
◉Resource needs: registry management, claims/EHR data partnerships, long-term follow-up processes.
◉Operational notes: integration with health systems and payer data sources is key.
2 By Study Design — operational tradeoffs
◉Interventional (RCTs, Platform/Basket)
◉Complexity: high (randomization, blinding, DSMBs).
◉Advantages: gold standard for efficacy.
◉Operational needs: randomization systems, clinical endpoint committees, central adjudication.
Observational (Registries, RWE)
◉Complexity: moderate, but data heterogeneity high.
◉Advantages: cost-effective, large sample sizes, long follow-up.
◉Operational needs: data harmonization, ETL pipelines, cleaning & provenance.
Expanded Access
◉Complexity: regulatory & compassionate use procedures.
◉Operational needs: close safety reporting, ethical oversight, logistics for off-label distribution.
3 By Indication — key operational challenges & strategic levers
◉Oncology
◉Challenge: biomarker stratification, rapid standard-of-care changes, competition for patients.
◉Levers: basket/platform trials, centralized NGS testing, partnerships with academic centers.
CNS
◉Challenge: subjective endpoints, slow progression, high placebo effects.
◉Levers: digital biomarkers (speech, gait sensors), long-term passive monitoring, enriched enrollment strategies.
Cardio/Metabolic
◉Challenge: event-driven endpoints require long follow-up and large N.
◉Levers: surrogate biomarkers, pragmatic trial designs, EHR-based event capture.
Rare/Orphan
◉Challenge: tiny populations, variable natural history.
◉Levers: adaptive designs, n-of-1, external/synthetic controls, accelerated approvals & orphan incentives.
4 By Service Type — operational considerations & vendor selection criteria
◉CRO Services
◉What to evaluate: global footprint, therapeutic experience, data quality record, and regulatory inspection history.
◉Operational need: integrated CTMS/EDC, vendor oversight processes.
Site Network Management
◉What to evaluate: investigator productivity, patient registries, contracting speed.
◉Operational need: SLA for site activation, eTMF completeness.
◉eClinical Tech (EDC, eConsent, ePRO)
◉What to evaluate: compliance (21 CFR Part 11), integration capability with wearables/EHR, user adoption.
◉Operational need: validation plans, training programs, device management SOPs.
Supplies & Logistics
◉What to evaluate: cold-chain capabilities, regional distribution partners, comparator sourcing.
◉Operational need: temperature mapping, contingency routes, chain-of-identity documentation.
Analytics & RWE
◉What to evaluate: data provenance, methods for bias adjustment, regulatory acceptance track record.
◉Operational need: ETL standards, synthetic control development SOPs, explainability for ML models.
Top 5 FAQs
Q1: What is the current size and projected size of the global clinical trials market?
A: The market was estimated at USD 54.39 billion in 2024 and is projected to reach USD 94.68 billion by 2034, growing at a 5.7% CAGR (2024–2034). (User data)
Q2: How has COVID-19 affected clinical trial operations long-term?
A: COVID-19 accelerated decentralized/hybrid trial adoption, telemedicine, remote monitoring and digital endpoints. Many of these operational models persisted because they improve recruitment, retention and cost efficiency. (User data + examples such as ACTIV program.)
Q3: How many clinical trials/records exist in public registries?
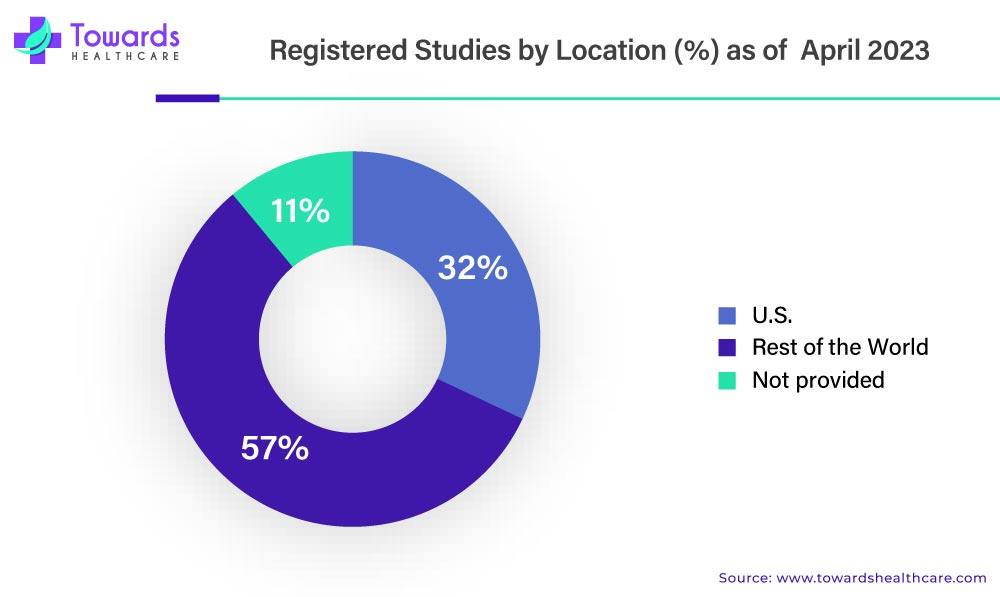
A: Public registries like ClinicalTrials.gov report hundreds of thousands of registered studies across 200+ countries (ClinicalTrials.gov historically >440k entries), reflecting large global trial throughput.
Q4: What are the biggest growth drivers for the clinical trials market?
A: Key drivers include rising R&D investments (biologics, gene/cell therapies), personalized medicine demands (biomarkers), regulatory acceleration programs (e.g., FDA CTAP), and adoption of digital health and decentralized trial models. (User data)
Q5: Which company types are dominating the market and why?
A: Large full-service CROs (IQVIA, Parexel, PPD) dominate because they offer integrated global services, data assets, regulatory expertise and end-to-end capabilities; specialized niche vendors provide decentralized trial tech, digital endpoints and AI analytics.
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5029
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Powering Healthcare Leaders with Real-Time Insights: https://www.towardshealthcare.com/healthcare-intelligence-platform
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest