The global life science market was about USD 88.2 billion in 2024, grew to USD 98.63 billion in 2025, and is projected to reach USD 269.56 billion by 2034 — a 2.73× increase (USD +170.93B) from 2025 to 2034 at a CAGR of 11.82% (2025–2034).
Download the free sample and get the complete insights and forecasts report on this market @ https://www.towardshealthcare.com/download-sample/5711
Market size
Key headline numbers & math
Reported values (source):
◉2024: USD 88.2 billion (reported as “US$ 88.2” in the source — interpreted as billion).
◉2025: USD 98.63 billion.
◉2034 (projection): USD 269.56 billion.
◉Absolute change (2025 → 2034): USD +170.93 billion (269.56 − 98.63).
◉Multiple (2025 → 2034): 2.73× (269.56 / 98.63 ≈ 2.733).
◉Annualized growth: CAGR ≈ 11.82% (the source-stated CAGR for 2025–2034; when applied, it reproduces the 2034 projection).
◉Single-year growth (2024 → 2025): 11.83% ((98.63 / 88.2) − 1).
◉Implication: the market more than doubles in a decade (2025–2034) driven by biotech/AI adoption, higher R&D spend, and faster product approvals in several segments.
Concentration & scale notes
◉The market figures represent a broad ecosystem (pharma, biotech, devices, tools, digital health, AI). The headline total therefore aggregates high-margin biologics alongside volume-driven reagents & consumables.
◉Because the life science market mixes product (devices, drugs) and services/platforms (CDMOs, CROs, analytics), value creation is uneven: high-value biologics and platform/software segments grow faster (% terms) than commoditized consumables.
Market trends
Macro-level structural trends
Rising R&D intensity
◉R&D investments and new drug discovery are primary growth engines; CRO/CDMO R&D spending grew ~12–13% annually (2014–2022, per cited McKinsey data in your content).
◉NIH (U.S.) remains a major public funder (USD 48 billion annually), supporting translational research pipelines.
Consolidation of biotech & startup momentum
◉Venture flows: India VC USD 13.7B into life sciences (2025 data), Canada recorded USD 12B across 65 deals in 2023 — strong VC activity is fueling startups and scale-ups.
Regulatory evolution & incentives
◉China’s Dec 2024 policy incentives to ease market access and clinical trial pilots — these accelerate launches in Asia.
◉The European Commission planning a “Strategy for European Life Sciences” to foster green/digital transitions.
Clinical trial expansion
◉Clinical trials footprint large: 540,338 trials registered on ClinicalTrials.gov (as of June 2025) — more trials equals larger demand for services, analytics, trial tech, CRO capacity.
Technology & commercialization trends
AI & data platforms rising rapidly
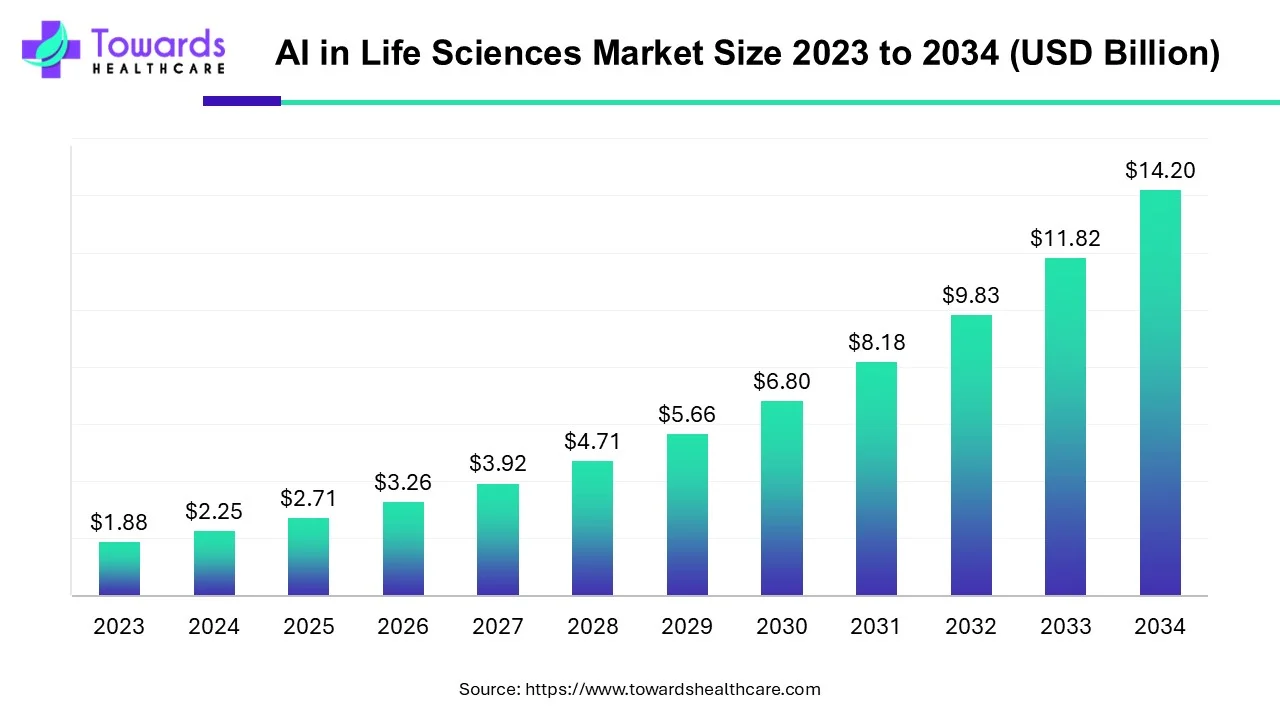
◉AI in life sciences market: USD 2.25B (2024) → USD 2.71B (2025) → USD 14.20B (2034); CAGR 20.21% (2024–2034).
◉Corporate examples: Accenture + 1910 Genetics (Oct 2024) to accelerate target ID; Axtria developing GenAI for commercial/clinical optimization.
Digital manufacturing & operations
◉Honeywell’s April 2025 TrackWise Manufacturing (AI-assisted, cloud-native) shows focus on bridging digital/physical manufacturing for life sciences — operational AI for compliance & throughput.
Cross-border partnerships & national trade pushes
◉Austrade + AusBiotech (June 2025) and BIOQuébec + Life Sciences BC (May 2025) are formalizing export/growth channels — national trade & cluster diplomacy is a clear trend.
Regulatory & reimbursement pressure
◉With more products and companion diagnostics (188 FDA companion diagnostics approved as of March 2025), payers and regulators are tightening evidence requirements and lifecycle management.
Product approvals supporting momentum
◉50 new drug approvals in 2024 (32 NCEs + 18 biologics); 21 devices approved by FDA January–October 2024; 43 cell & gene therapies approved by Jan 2025 — all indicate active innovation-to-approval pipelines.
AI impact & role in the life-science market
1) Drug discovery & lead identification (core)
◉Value proposition: accelerate target ID, reduce candidate attrition, shorten discovery cycles, prioritize molecules with optimal ADMET profiles.
◉Mechanics: multi-omics integration (genomics, proteomics), structure-based models (physics-informed + ML), generative chemistry for novel scaffolds.
◉Industrial metrics: time-to-hit, number of prioritized leads, predicted vs. observed assay hit rate.
◉Real example from source: 1910 Genetics platform — targeted to accelerate drug target identification and cut costs (Accenture partnership).
2) Preclinical modelling & in-silico experiments
◉Use: simulate biological pathways, toxicity prediction, and virtual screening — reduces animal studies and early lab costs.
◉Risk/validation: needs robust external validation; regulatory acceptance requires clear model transparency.
3) Clinical trials (design, recruitment, monitoring)
◉Use-cases: adaptive trial design optimization, site selection using real-world data (RWD), patient recruitment predictions, remote monitoring via wearables.
◉Impact: higher enrollment speed, fewer protocol amendments, improved retention.
◉Scale indicator: with 540k trials registered, incremental AI adoption in trial operations generates sizeable efficiency gains.
4) Diagnostics & companion diagnostics
◉Role: AI-enabled image analysis, multi-modal biomarker interpretation, and predictive diagnostics that enable earlier detection and precision therapy selection.
◉Companion diagnostics: supporting targeted oncology approvals (188 companion diagnostics approved by FDA as of March 2025).
5) Manufacturing, quality & supply chain (operational AI)
◉Capabilities: predictive maintenance, batch quality prediction, anomaly detection, and regulatory traceability.
◉Example: Honeywell TrackWise Manufacturing — AI-assisted platform to align digital operations with physical manufacturing, reducing compliance burden and improving throughput.
6) Commercial & market access (GenAI)
◉Use: personalized HCP engagement, market segmentation, demand forecasting, and promotional optimization.
◉Example: Axtria’s GenAI solutions for outreach personalization and analytics-driven commercial strategies.
7) Post-market surveillance & pharmacovigilance
◉Use: NLP on EHRs / social media to detect safety signals faster, automated case processing, signal prioritization.
◉Regulatory note: PV algorithms must satisfy auditability and traceability.
8) Data infrastructure & ecosystems
◉Need: federated learning and privacy-preserving methods to unlock multi-center datasets while preserving patient privacy.
◉Constraint: data privacy and regulatory compliance are major bottlenecks.
9) Risks & mitigation
◉Risk: data bias, reproducibility, black-box models, regulatory acceptance, cybersecurity.
◉Mitigation: explainable AI, model registries, validation datasets, third-party auditing, and alignment to GxP.
10) KPIs for AI programs (what companies should measure)
◉time-to-insight, reduction in experimental cycles, predicted vs. actual validation hit rates, per-project cost savings, model explainability score, regulatory acceptance rate.
Regional insights
North America (lead region)
◉Why leading: deep VC ecosystems, world-class academic research, dense pharma/biotech clusters, and significant public funding (NIH ≈ USD 48B/year).
U.S. specifics:
◉R&D intensity: >45,000 life science companies in the U.S. (early 2024).
◉Approvals: 50 new drugs in 2024 (32 NCEs + 18 biologics) — accelerates commercial pipeline value.
◉Implication: Concentration of capital + regulatory proximity to FDA means faster commercialization and higher valuations for U.S. innovators.
Canada:
◉Scale: >2,000 life science companies; USD 25.9B invested (2019–2023); USD 12B in 65 deals in 2023 — strong niche VC/cluster momentum.
Asia-Pacific (fastest growth)
◉Drivers: expanding domestic markets, government incentives (China), increasing VC flows (India).
China:
◉Ecosystem: >3,000 life science companies; ~270k employees.
◉IP push: patent activity up (379% growth from 2014 per source); Shanghai & Beijing active VC centers (USD 1.7B VC activity in 2024).
◉Policy: Dec 2024 State Council incentives to cut regulatory hurdles and pilot clinical trial authorizations.
India:
◉Startups: from 5,365 → 8,531 (2021–2023).
◉VC: USD 13.7B life science VC (2025 report).
◉Implication: lower-cost clinical/research capabilities + growing domestic manufacturing make India both a growth and cost arbitrage hub.
Europe (steady, policy-driven)
◉Policy: European Commission strategy planning to boost green/digital transitions in life sciences.
◉Country notes: Germany — strong medtech innovation and per-capita healthcare spend (€5,832). UK — £21.7B pharma exports in 2024; LSIMF commitment £520M for manufacturing.
◉Implication: Europe attracts R&D and manufacturing investment when policy, talent, and incentives align.
Latin America
◉Clinical trials growth: Brazil leads region; 1,723 active/recruiting trials (as of June 6, 2025).
◉Implication: attractive for multi-site trials (cost + patient diversity), growing startup scene, regulatory harmonization remains a driver.
Middle East & Africa (emerging)
◉Funding & M&A: MEA governments allocating ~USD 10B for R&D; Q3 2024 M&A ~USD 1.8B (big Q-over-Q spike reported).
◉Implication: partnerships and capacity building (manufacturing/clinical) are the main near-term growth vectors.
Market dynamics
Drivers
◉New drug discovery & pipeline expansion — major responsible factor for market growth; high-value novel therapeutics (biologics/cell-gene) increase market value per product.
◉Increasing R&D spend — public (NIH ~USD 48B) and private capital fueling translational and clinical phases.
◉Advances in enabling technologies — genomics, proteomics, AI, digital health and manufacturing tech (e.g., Honeywell TrackWise) raise productivity.
◉VC & M&A — billions flowing into startups (India USD 13.7B; Canada USD 12B in 2023); M&A spikes (MEA example) signal consolidation and scale.
Restraints / headwinds
◉Regulatory compliance complexity — changing rules increase time-to-market and compliance costs across markets.
◉Data privacy & cybersecurity — digitization introduces breach risk and regulatory scrutiny.
◉Reimbursement pressure & payer evidence requirements — more companion diagnostics and precision therapies require stronger real-world evidence.
◉Talent and infrastructure mismatch in emerging regions — scale-up barriers despite VC inflows.
Opportunities
◉Genetic engineering & precision medicine — CRISPR & RNA therapeutics open up high-value rare disease and oncology opportunities.
◉AI-first products & platforms — AI market growth (CAGR ~20.21%) indicates commercializable platform opportunities across discovery, trials, and ops.
◉Telemedicine & digital therapeutics — >320 million users of health apps (2024) signals consumer adoption and commercial TAM for software-based interventions.
◉Geographic arbitrage & nearshoring — Asia-Pacific manufacturing & trial capacity grows; China/India policy pushes improve access.
Top companies

AstraZeneca
◉Overview: Global pharmaceutical company with core strengths in oncology, cardiovascular, metabolic, respiratory, and immunology.
◉Products: Branded therapeutics across oncology and biologics.
◉Strengths: Large late-stage pipelines, global commercialization reach, strong oncology franchise.
Baker Tilly
◉Overview: Professional services and consulting; Life Sciences consulting practice noted in your content.
◉Products/offerings: StewardshipNOW (new program), compliance & funding strategy services.
◉Strengths: Compliance expertise, partnerships (e.g., with MediSpend) to streamline external funding and reduce administrative burdens.
BIOQuébec
◉Overview: Industry association supporting Quebec life science ecosystem.
◉Strengths: Regional cluster development, facilitating MoUs and cross-border collaborations (e.g., with Life Sciences British Columbia).
Clarivate plc
◉Overview: Data & analytics provider for life sciences.
◉Product: DRG Fusion (commercial analytics platform).
◉Strengths: Deep data assets, analytics for commercial strategy and market intelligence; reduces raw data complexity for product positioning.
Eli Lilly & Company
◉Overview: Major global pharma, strong in diabetes, oncology, immunology.
◉2024 figures (source): Q4 2024 revenue USD 13.53B; FY 2024 revenue USD 45.042B.
◉Strengths: Robust clinical pipeline, strong commercial execution, recent revenue growth noted in source.
GlaxoSmithKline (GSK)
◉Overview: Broad pharmaceutical and vaccine portfolio; strong presence in vaccines and consumer healthcare (historically).
◉Strengths: Vaccine pipeline, global manufacturing footprint, established commercial channels.
Honeywell
◉Overview: Industrial technology conglomerate; moving into life sciences operational software.
◉Product: TrackWise Manufacturing (AI-assisted, cloud native).
◉Strengths: Industrial controls and compliance expertise, ability to scale manufacturing-quality systems.
Merck KGaA (The Merck Group)
◉Overview: German multinational active in life science tools, pharma, and performance materials.
◉2024 figures (source): Q4 2024 net sales USD 15.6B; FY 2024 USD 64.2B.
◉Strengths: Broad portfolio spanning tools & reagents to pharma, strong European base.
Novartis
◉Overview: Global pharma with big oncology and innovative therapy footprints.
◉Strengths: Strong R&D, scale manufacturing, and commercialization.
Novo Nordisk
◉Overview: Leader in diabetes/GLP-1 therapies.
◉Strengths: Market leadership in metabolic disease therapeutics and scale in global supply chains.
Pfizer
◉Overview: Broad pharma with recent high visibility (vaccines) and strong commercial muscle.
◉Strengths: Global reach, manufacturing, established regulatory relationships.
Roche
◉Overview: Diagnostics + pharma integrated model; leader in oncology diagnostics and therapeutics.
◉Strengths: Companion diagnostics leadership (complements therapeutic approvals).
Schrödinger, Inc.
◉Overview: Software & platform company for chemical simulation targeting drug discovery.
◉2024 figures (source): Q4 2024 revenue USD 88.3M; FY 2024 revenue USD 207.5M.
◉Strengths: Physics-informed molecular simulation, tooling used by pharma for computational chemistry.
Thermo Fisher Scientific
◉Overview: Market leader in life-science instruments, consumables, and services.
◉Strengths: Instrumentation scale, reagent & consumables leadership, global service networks — strong position in research and manufacturing workflows.
Latest announcements
Austrade + AusBiotech (June 2025)
◉What: Collaboration to accelerate international expansion of Australian life science firms; creation of a National TradeStart Adviser.
◉Impact: improves export readiness, connects firms to trade programs, and boosts cluster internationalization.
BIOQuébec + Life Sciences British Columbia (May 2025)
◉What: MoU to accelerate cross-regional life science innovation, investment, and commercialization.
◉Impact: cross-province resource exchange, joint commercialization pathways, boosted investor networks.
Prudentia Sciences funding (Jan 2025)
◉What: USD 7M raised to provide data-driven decision platforms for biopharma portfolio and pipeline acceleration.
◉Impact: supports investor decision-making and portfolio strategies to de-risk investments in drug pipelines.
Accenture × 1910 Genetics (Oct 2024)
◉What: Partnership & investment to provide tailored solutions + scalable infrastructure for target identification.
◉Impact: Shortens discovery timelines; brings enterprise IT scale to genomics-driven discovery.
Baker Tilly — stewardshipNOW launch
◉What: Platform to help life science companies manage external funding in a compliant, ethical way (in partnership with MediSpend).
◉Impact: reduces administrative overhead and mitigates compliance & risk for funded programs.
Honeywell — TrackWise Manufacturing (Apr 2025)
◉What: AI-assisted, cloud-native platform for manufacturing & compliance workflows.
◉Impact: bridges digital/physical manufacturing, streamlines regulatory documentation, increases production flexibility.
Clarivate — DRG Fusion (Jan 2025)
◉What: New commercial analytics platform for biopharma & medtech to simplify disease and competitive landscapes.
◉Impact: helps identify product positioning gaps and commercialization opportunities.
Recent developments
Policy & regulation changes
◉China (Dec 2024) incentivizing life sciences — immediate effect: accelerating local launches and foreign access.
◉WHO Technical Advisory Group on responsible life sciences use — indicates elevated global governance focus on dual-use/biorisk.
Platform & software launches
◉Honeywell TrackWise and Clarivate DRG Fusion signal a move toward end-to-end digital workflows (manufacturing compliance → commercial analytics).
Funding & partnerships
◉Prudentia $7M and Accenture’s engagement with 1910 Genetics indicate private capital targeting tools to accelerate discovery & investment decisions.
Clinical & approval momentum
◉FDA and approvals data (50 new drugs in 2024; device approvals; 43 cell/gene approvals by Jan 2025) indicate sustained regulatory throughput; companion diagnostics (188 approvals as of Mar 2025) show precision medicine commercial integration.
Regional trade & cluster strengthening
◉Austrade/AusBiotech and BIOQuébec collaborations show policy-level cluster support to convert R&D into exports; expect faster market entry for partnered firms.
Segments covered
By Type
Pharmaceuticals
◉Subsegments: branded drugs, generic drugs, biosimilars.
◉Explanation: high-value prescription drugs dominate revenue; generics/biosimilars add volume-based revenue and pricing pressure.
Biotechnology
◉Subsegments: genomics, proteomics, metabolomics, bioinformatics.
◉Explanation: driving biologics, cell & gene therapies, and enabling precision therapeutics via omics.
Medical Devices
◉Subsegments: diagnostics, therapeutic devices, wearables.
◉Explanation: device evolution (AI-enabled diagnostics, wearables) increases recurring data + device-as-platform revenue.
Life Science Tools
◉Subsegments: instruments, reagents & consumables, analytical tools.
◉Explanation: backbone of laboratory workflows — predictable recurring revenue, margin variability.
Digital Health Solutions
◉Subsegments: AI in life sciences, cloud platforms, health informatics.
◉Explanation: platform economics, scalable software margins, and cross-selling into R&D/commercial ops.
By Application
Therapeutics
◉Explanation: largest revenue contributor because approved therapies command high price points and long-term use.
Drug Discovery & Development
◉Explanation: fastest CAGR — demand for discovery platforms, CRO/CDMO services, and AI-enabled tools.
Diagnostics & Clinical Trials
◉Explanation: diagnostics growth driven by screening programs, companion diagnostics growth; trials growth increases demand for trial tech and CRO services.
By Therapeutic Areas
Oncology (dominant in 2024)
◉Explanation: intensity of R&D, high-value biologics, and companion diagnostics drive revenue.
Infectious Diseases (fastest growth forecast)
◉Explanation: renewed emphasis on pandemic preparedness, vaccine innovation, and antivirals.
Cardiology, Neurology, Immunology, Rare Diseases
◉Explanation: demographic trends (aging) and biologic innovation drive investment.
By Region
Detailed in the Regional Insights section above.
Top 5 FAQs
1) What is the current size and projected size of the global life science market?
◉Answer: The market was USD 88.2B in 2024, USD 98.63B in 2025, and is projected to reach USD 269.56B by 2034 (CAGR 11.82% from 2025–2034).
2) Which region led the market in 2024 and which will grow fastest?
◉Answer: North America led in 2024 (U.S. strength, NIH funding USD 48B). Asia-Pacific is expected to be the fastest-growing region over the forecast period driven by China and India.
3) How big is AI in the life sciences and why does it matter?
◉Answer: AI in life sciences was USD 2.25B in 2024, USD 2.71B in 2025, and is projected at USD 14.20B by 2034 (CAGR 20.21%). AI shortens discovery cycles, automates trials, improves diagnostics, and optimizes manufacturing & commercial functions — so it multiplies productivity across the value chain.
4) What are the principal market restraints?
◉Answer: Major restraints include regulatory compliance complexity, data privacy & cybersecurity, and payer/reimbursement pressures. These increase cost and time-to-market for products and digital solutions.
5) Who are the top players and what are their strengths?
◉Answer: Top players listed in your content include AstraZeneca, Eli Lilly, Merck KGaA, Novartis, Pfizer, Roche, Thermo Fisher, Schrödinger, and others. Strengths vary from large-scale R&D & commercial channels (big pharmas) to platform/software specialization (Schrödinger) and instrumentation & consumables scale (Thermo Fisher). Example financial anchors: Merck Group FY 2024 sales USD 64.2B, Eli Lilly FY 2024 USD 45.042B, Schrödinger FY 2024 USD 207.5M.
Access our exclusive, data-rich dashboard dedicated to the life science sector – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5711
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Powering Healthcare Leaders with Real-Time Insights: https://www.towardshealthcare.com/healthcare-intelligence-platform
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest