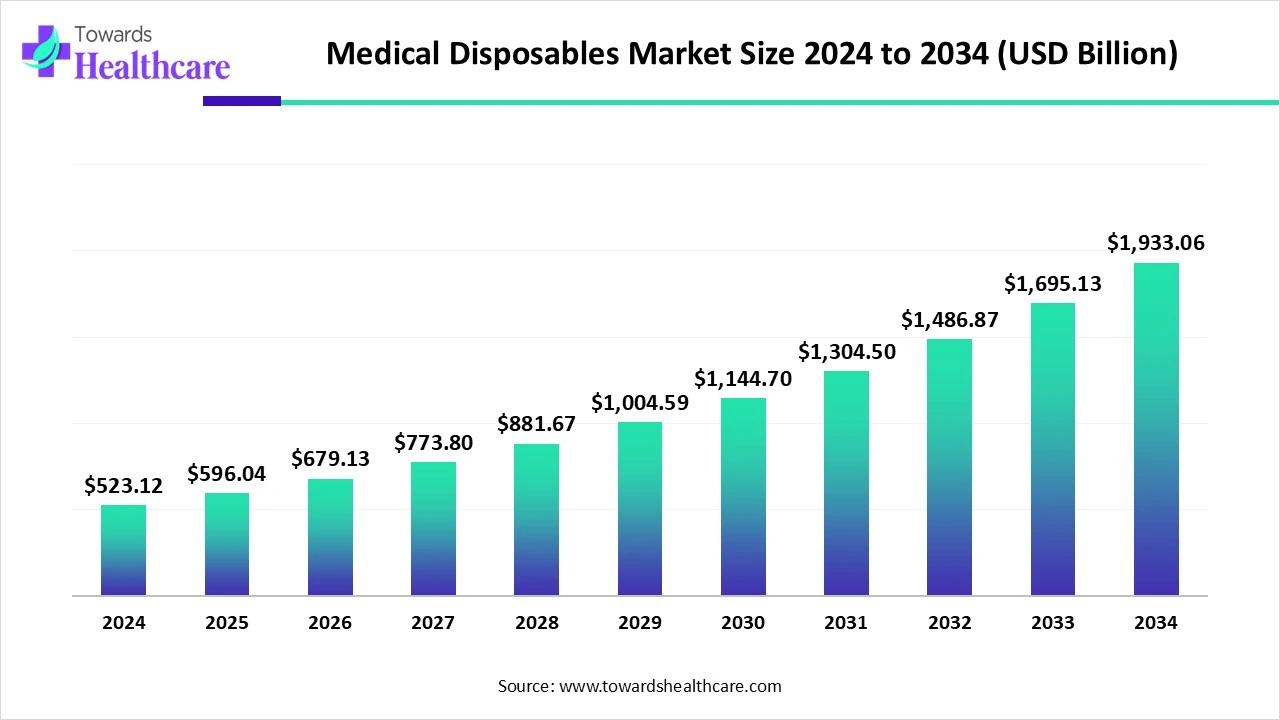
The global medical disposables market was USD 523.12 billion in 2024, is estimated at USD 596.04 billion in 2025 (a USD 72.92 billion increase year-on-year), and is projected to reach USD 1,933.06 billion by 2034 a forecast CAGR of 13.94% (2025–2034).
Download this Free Sample Now and Get the Complete Report and Insights of this Market Easily @ https://www.towardshealthcare.com/download-sample/6086
Market size
Base / recent values
◉2024 market: USD 523.12B.
◉2025 estimate: USD 596.04B (increase of USD 72.92B vs 2024).
◉2034 projection: USD 1,933.06B.
Forecast growth rate
◉CAGR 2025–2034: 13.94% (implies a near-quadrupling of market value across the decade).
◉Implicit scale & composition indicators (2024 reference shares → implied values)
◉North America (38% of 2024) → ≈ USD 198.79B (2024).
◉PPE & infection-control consumables (28–32% share in 2024) → ≈ USD 146.47B–167.40B.
◉Single-use polymers/plastics (≈60% share in 2024) → ≈ USD 313.87B.
◉Hospitals & acute care (≈62% share in 2024) → ≈ USD 324.33B.
◉Sterile procedural consumables (≈48% share in 2024) → ≈ USD 251.10B.
◉GPO/direct OEM contracts (≈55% share in 2024) → ≈ USD 287.72B.
Interpretation
◉Large absolute base with concentration in a few high-value segment buckets (polymers, hospital use, sterile consumables) — meaning incremental demand or price shifts in those buckets materially change the market total.
◉Rapid projected expansion implies significant new product introductions, supply-chain scaling, and channel shifts (e-commerce, DTP, managed services).
Market trends
Infection-control and PPE remain foundational
◉PPE & infection-control consumables held 28–32% in 2024 — reflecting sustained infection-prevention priorities post-COVID and ongoing hospital infection control programs.
Shift to single-use plastics / polymers
◉Single-use polymers/plastics dominated (60% share in 2024) due to cost, sterilization logistics, and pre-sterilized packaging benefits.
Fastest growing product pockets
◉Catheters & vascular access expected to be the fastest growing product category (2025–2034) — driven by aging populations, more minimally invasive procedures, and rising chronic disease prevalence.
◉Advanced polymer dressings & hydrocolloids expected to register fastest material/technology growth — tied to wound-care performance and outpatient wound-management trends.
Care-setting shifts
◉Hospitals/acute care dominated in 2024 (62%) but ambulatory surgery centers/outpatient clinics are the fastest growing care settings as procedures migrate out of hospitals.
◉Sterile vs non-sterile dynamics
◉Sterile procedural consumables commanded the largest value (48%) in 2024, while non-sterile patient care consumables are forecast to grow fastest — reflecting scale in lower-complexity, high-volume items (gloves, basic disposables).
Distribution & commercial model evolution
◉GPO/direct OEM dominated in 2024 (55%) because of bulk purchasing and cost containment; e-commerce/B2B marketplaces expected to be the fastest growing channel as digital procurement penetrates healthcare.
Geographic shifts
◉North America leads (38% in 2024) on advanced care, installed base, and reimbursement; Asia-Pacific is the fastest-growing region driven by rising healthcare spend, home healthcare adoption, and local manufacturing.
Policy & local manufacturing tailwinds
◉Government initiatives (example: India, Jan 2024) boosting local production of disposables, altering regional supply chains and costs.
Product-bundle and kit strategies
◉Increasing use of pre-assembled single-use kits (e.g., hysteroscopy kit examples) to simplify outpatient procedures and reduce set-up time.
AI role / impact on the medical disposables market
Demand forecasting & inventory optimization (operational AI)
◉AI models analyze historical utilization, seasonal trends, and epidemiological signals to reduce stockouts and overstocking for high-volume disposables (syringes, PPE).
◉Improved GPO and hospital forecasting reduces working capital and waste from expired disposables.
Design acceleration & materials discovery
◉Generative ML models and materials informatics speed development of next-gen polymers, biodegradable materials and advanced dressings (hydrocolloids) by predicting performance and manufacturability.
◉Shortens R&D cycles for product iterations and tailored wound-care dressings.
Quality control & defect detection (manufacturing AI)
◉Computer vision inspects molding, packaging seals, and sterility indicators at line-speed — lowering defect rates and ensuring compliance for sterile procedural consumables.
Process optimization & yield improvement
◉Reinforcement learning and advanced analytics tune extrusion, molding and sterilization processes to increase throughput for single-use plastics and lower per-unit costs.
Regulatory intelligence & submission automation
◉NLP systems help compile technical files, map requirements across jurisdictions, and track regulatory changes — accelerating approvals for disposables destined for multiple markets.
AI in product usage and clinical decision support
◉Embedded analytics for single-use diagnostic cartridges and point-of-care kits can triage results, reducing unnecessary consumable usage by guiding clinicians to high-value tests.
Smart disposables & traceability
◉Low-cost sensors + AI provide usage, temperature history, and chain-of-custody for sterile kits — enabling just-in-time usage and recall management.
Customer segmentation and commercial targeting
◉Predictive models identify high-value accounts (ASCs, large hospital systems) and customize contracting strategies (GPO/OEM vs DTP) to improve channel ROI.
Sustainability optimization
◉AI models evaluate life-cycle tradeoffs between disposable and reusables (energy, water, carbon footprints) to inform corporate sustainability strategies and product roadmaps.
Clinical outcomes & value demonstration
◉AI links real-world outcomes with disposable product usage (e.g., infection rates vs specific drape types), enabling better reimbursement and premium positioning for higher-value disposables.
Risks & governance
◉AI adoption also creates new risks: data privacy, black-box decisioning for clinical guidance, and need for validation/traceability — manufacturers must govern models used in clinical contexts.
Regional insights
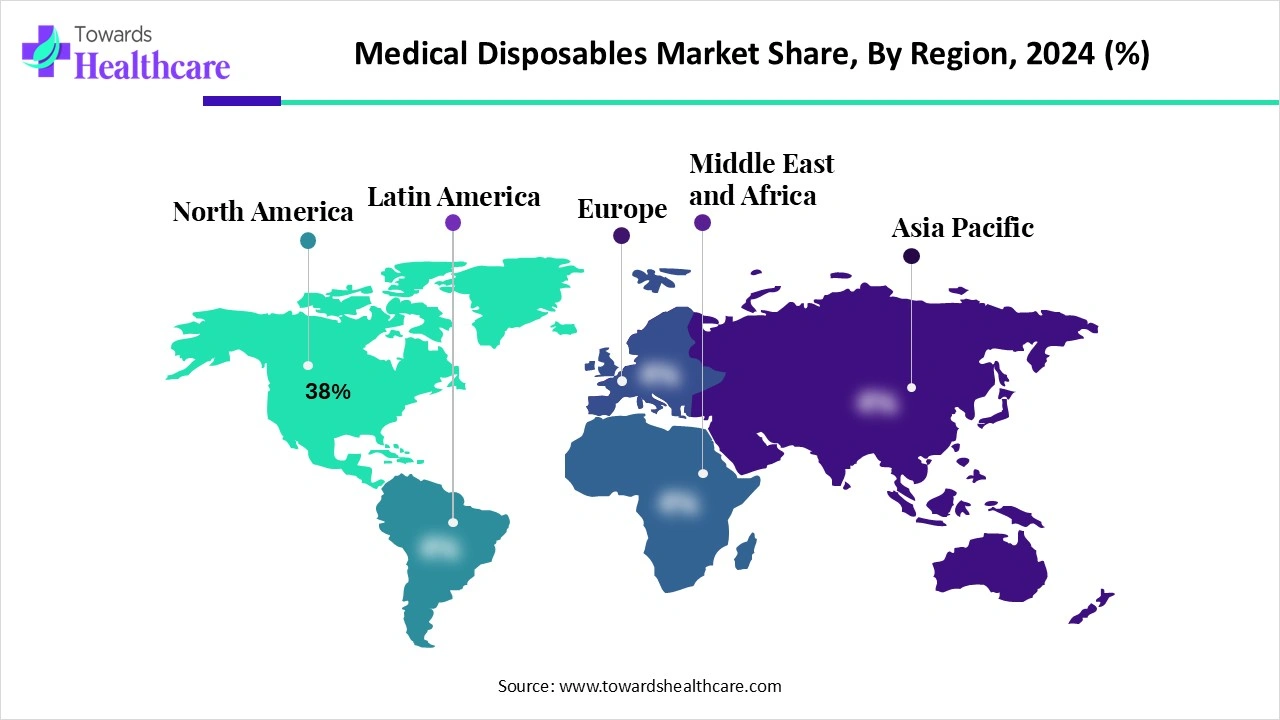
North America (dominant 38% in 2024)
Market drivers
◉High procedural volumes, advanced hospital infrastructure, extensive adoption of new medical devices.
◉Strong reimbursement frameworks and higher per-procedure spend on disposables.
Supply-chain & commercialization
◉Large GPO presence drives centralized procurement (explains 55% GPO share).
◉Demand for integration with hospital ERP/EHR systems and traceability.
Innovation & regulatory environment
◉Fast uptake of advanced disposables (single-use endoscopes, advanced dressings) and rapid regulatory pathways for iterative improvements.
Value implication
◉High willingness to pay for convenience and infection-control, giving premium pricing potential.
Asia-Pacific (fastest growing)
Drivers
◉Expanding healthcare access, rising income, aging populations, and increased home healthcare adoption.
◉Government manufacturing incentives (e.g., India) increasing local production of disposables.
Market dynamics
◉Cost sensitivity pushes for local sourcing & private-label converters, but rising clinical standards push premiumization in urban centers.
Opportunities
◉Scale manufacturing and export hubs; targeted growth for catheters, single-use polymers, and wound dressings.
Europe
Drivers
◉Regulatory stringency, focus on sustainability, and high standards for infection control.
Market nuances
◉Growing interest in sustainable/biodegradable disposables and circular-economy pilots; payers challenge prices but value safety and environmental claims.
Latin America / MEA
Challenges & potential
◉Fragmented procurement, lower per-capita spend, but high unmet need in many markets.
◉Opportunity for low-cost disposables, local partnerships, and donor/NGO-supported programs.
Country-level callouts (examples from content)
◉U.S.: leadership in device approvals and high adoption, reliant partly on international suppliers but strong in innovation.
◉India: government initiatives (Jan 2024) to boost local device manufacturing—important for disposable categories (catheters, cannulas, syringes).
Market dynamics
Drivers (why the market expands)
◉Infection prevention priority — PPE & infection control largest product bucket (28–32%).
◉Higher procedure volumes & outpatient shift — drives need for pre-sterilized, single-use kits.
◉Aging population & chronic disease — fuels demand for catheters, vascular access, wound care.
◉Convenience & logistics — single-use avoids sterilization costs and reduces cross-contamination risk (single-use polymers ≈60%).
◉Commercial consolidation — GPOs and OEM contracts centralize purchases and bring scale.
Restraints (what limits growth)
◉Regulatory complexity — strict safety standards increase time-to-market and compliance costs (explicit restraint in provided content).
◉Environmental & sustainability pressure — single-use plastics face scrutiny; potential policy/regulatory headwinds or additional costs for biodegradable alternatives.
◉Price sensitivity in emerging markets — can limit adoption of higher-margin disposables unless local cost structures are optimized.
Opportunities (areas to capture upside)
◉Next-gen disposable instruments — disposable forceps, blades and single-use devices to replace reusable items where sterilization costs are high.
◉E-commerce & DTP channels — rapid growth opportunity as procurement digitizes.
◉AI & smart disposables — value capture via traceability, outcomes data and premium pricing.
◉Local manufacturing & private-label — countries with policy support (e.g., India) open scalable production and export potential.
Implications for stakeholders
◉Manufacturers: invest in scale, automation, and advanced materials; diversify channels (GPO + e-commerce).
◉Hospitals / GPOs: optimize inventory with predictive analytics; balance cost vs infection-control outcomes.
◉Policymakers: need to balance local manufacturing incentives with environmental regulations.
Top companies

3M
◉Products: Wide portfolio including wound care dressings, surgical tapes, sterilization products, adhesives, PPE.
◉Overview: Broad healthcare consumables and materials footprint.
◉Strengths: Diversified product mix, scale manufacturing, distribution relationships, strong R&D in materials.
Medline Industries
◉Products: Comprehensive disposables catalog — gowns, gloves, procedure kits, wound care, infection control.
◉Overview: Major medical distributor and manufacturer focused on consumables.
◉Strengths: Deep distribution, private-label capabilities, close hospital relationships, inventory/managed services.
Cardinal Health
◉Products: Surgical gloves, procedure kits, distribution and logistics services.
◉Overview: Large healthcare services & distribution platform with disposable offerings.
◉Strengths: Supply-chain scale, GPO contracts, logistics and managed inventory expertise.
Becton, Dickinson & Company (BD)
◉Products: Syringes, safety needles, IV sets, diagnostic disposables.
◉Overview: Leader in safety devices and diagnostic consumables.
◉Strengths: Clinical trust in safety devices, broad clinical adoption, regulatory experience.
Baxter International
◉Products: Infusion systems, IV/infusion sets, single-use components for renal and infusion care.
◉Overview: Focused on infusion and renal disposables and therapy solutions.
◉Strengths: Therapy-focused products, integrated solutions and service models.
B. Braun Melsungen
◉Products: Vascular access, infusion therapy, surgical disposables.
◉Strengths: Strong clinical portfolio, global presence in hospital consumables.
Kimberly-Clark Health / Halyard Health
◉Products: Surgical gowns, procedure packs, infection control PPE.
◉Strengths: Brand recognition in PPE and clinician apparel; scale in hygiene products.
Ansell
◉Products: Protective gloves and PPE.
◉Strengths: Specialty in protective wear and infection control.
Smiths Medical
◉Products: Vascular access, infusion systems, monitoring disposables.
◉Strengths: Niche specialty devices and clinical adoption in critical care.
Terumo Corporation
◉Products: Catheters, vascular access devices, infusion sets.
◉Strengths: Innovation in minimally invasive disposables and catheter technologies.
Mölnlycke Health Care
◉Products: Advanced wound dressings, single-use surgical products.
◉Strengths: Strong position in wound care, advanced dressing tech.
ConvaTec, Coloplast, Fresenius Kabi, Owens & Minor, Henry Schein, Nipro, Teleflex, Cook Medical
◉Products/Strengths: Mix of wound care, ostomy, infusion/renal consumables, distribution services, catheter and interventional disposables — each brings niche leadership, OEM relationships, or distribution strength.
Latest announcements
Coherent Corp. — June 2025
◉Announcement: Launch of a new line of disposable surgical fiber assemblies for precision medical applications; suitable for OEM integration and direct clinical use.
◉Implications: Entry of photonics/specialty components into disposables expands OEM supply options for single-use optical/laser assemblies used in precision surgery; may enable new disposable device classes and simplify OR logistics.
Microbot Medical Inc. — June 2025
◉Announcement: Expansion of commercial team in preparation for anticipated U.S. launch of the LIBERTY Endovascular Robotic System, with an expected FDA 510(k) decision in Q3 2025.
◉Implications: Robotic systems often require specific single-use disposables (catheters, sheaths) — increased commercialization activity signals potential near-term demand lift in endovascular disposables.
Johnson & Johnson — June 2025
◉Announcement: Launch of ACUVUE OASYS MAX 1-Day MULTIFOCAL for ASTIGMATISM — the first daily disposable contact lens addressing astigmatism + presbyopia.
◉Implications: Expands the daily disposable contact lens category and underlines growth in single-use ophthalmic disposables; potential to shift eye-care consumable spending patterns.
Minerva Surgical — May 2025
◉Announcement: HERizon Hysto-Kit launch — a single-use, pre-assembled kit for in-office hysteroscopy.
◉Implications: Illustrates trend toward procedure-specific single-use kits simplifying outpatient workflows and reducing setup time; directly targets ambulatory/outpatient growth.
Fresenius Medical Care (FME) — June 2025
◉Announcement: Revealed new FME Reignite strategy at Capital Markets Day (London).
◉Implications: Strategic repositioning by a dialysis/renal leader can affect consumable procurement for renal disposables and service bundles.
KYRA Medical, Inc. — April 2025
◉Announcement: Opening a UK subsidiary to strengthen presence in UK/Europe and improve supply-chain efficiency.
◉Implications: Regional expansion to reduce lead times and bolster European supply of positioning/OR disposable products.
Henry Schein — November 2024
◉Announcement: Agreement to acquire Acentus, a national medical supplier specializing in delivery of Continuous Glucose Monitors (CGMs).
◉Implications: Strengthens Henry Schein’s disposable/diagnostic portfolio and DTP distribution for diabetes monitoring consumables.
Recent developments
◉Pre-assembled single-use kits gaining traction (Minerva HERizon) — supports outpatient procedural growth and reduces clinical setup time and variability.
◉Specialized disposable components entering new domains (Coherent’s disposable fiber assemblies) — expands definition of disposables beyond plastics to include precision photonic assemblies.
◉Commercial launches tied to advanced systems (Microbot’s LIBERTY) — signals adjacent disposable demand (robotic system single-use interfaces).
◉Regional expansion and supply-chain localization (KYRA UK subsidiary; India policy moves) — improves resiliency and shortens lead times; supports cost optimization and export capability.
◉Consolidation and M&A in distribution & diagnostics (Henry Schein + Acentus) — expands access to DTP and chronic care consumables (CGMs), illustrating cross-pollination between disposables and diagnostics.
◉Strategic corporate repositioning (Fresenius Reignite) — large therapy providers aligning strategies that will change procurement patterns for disposables in renal and chronic care.
Segments covered
By Product Category
◉PPE & Infection-Control Consumables: gowns, masks, face shields, procedure packs; dominant due to infection control priorities and regulatory mandates.
◉Syringes, Needles & Safety Devices: single-use injection devices with safety features to prevent needlestick injuries; high volume, regulated.
◉IV / Infusion Sets & Accessories: infusion tubing, connectors — essential for inpatient and home infusion therapies.
◉Catheters & Vascular Access: peripheral/central catheters, introducers — fastest-growing due to aging and minimally invasive interventions.
◉Wound Care Dressings & Advanced Interfaces: foam, hydrocolloid, alginate, advanced polymer dressings — rising due to chronic wounds and outpatient wound management.
◉Surgical Consumables & Procedure Kits: drapes, staplers, single-use scopes, pre-assembled kits — enable OR efficiency.
◉Diagnostic & Sample Collection: swabs, collection tubes, cartridges — critical for POC testing and lab workflows.
◉Single-use Endoscopes & Procedure Scopes: disposable endoscopes reducing sterilization cost and cross-contamination risk.
◉Other disposables: cannulas, feeding tubes, catheters, specialty single-use instruments.
By Material / Technology
◉Single-use Polymers / Plastics: dominant; low cost, mass producible, pre-sterilizable.
◉Non-woven textiles: gowns, drapes—single-use comfort and barrier properties.
◉Advanced polymer dressings & hydrocolloids: higher performance wound care materials.
◉Specialty biocompatible materials: for catheters, implants in single-use form factor.
◉Sustainable/biodegradable disposables: nascent but growing as regulatory/environmental pressure rises.
By Care Setting / End User
◉Hospitals & Acute Care: major share due to procedure density and acute care needs.
◉Ambulatory Surgery Centers & Outpatient Clinics: fastest growth — migration of procedures out of hospitals.
◉Home Healthcare & Infusion at Home: rising demand for infusion sets, wound care, DTP.
◉Long-term Care & Nursing Homes: steady consumption of PPE and routine disposables.
◉Diagnostic & Reference Labs: high volume of sample collection disposables.
By Sterility / Application
◉Sterile Procedural Consumables: kits and items for sterile procedures—dominant value.
◉Non-sterile Patient Care Consumables: gloves, basic supplies—fastest growth in volume.
◉Single-use Diagnostic Cartridges & Test Kits: POC and lab cartridge-based growth.
By Distribution Channel & Commercial Model
◉GPO / Direct OEM Contracts: dominant procurement method for hospitals (bulk purchasing, cost containment).
◉Medical Distributors & Wholesalers: support reach into community hospitals and clinics.
◉E-commerce / B2B Marketplaces: fastest growing channel for convenience and reach.
◉Direct-to-Patient / Home Delivery: growth in chronic care and home infusion.
◉Managed Inventory / Device-as-a-Service bundles: outcome-based contracting and service models for disposables + devices.
Top 5 FAQs
Q1 : What is the market size and growth rate for medical disposables?
A: The market was USD 523.12B in 2024, is USD 596.04B in 2025, and is projected to reach USD 1,933.06B by 2034, implying a CAGR of 13.94% (2025–2034).
Q2 : Which product categories and materials dominated the market in 2024?
A: PPE & infection-control consumables led product categories
Q3 : Which regions are leading and which are fastest growing?
A: North America dominated in 2024 (38% share). Asia-Pacific is the fastest-growing region for the forecast period (2025–2034).
Q4 : What are the biggest commercial channels and shifts?
A: GPO/direct OEM contracts dominated (55% in 2024) due to bulk procurement; e-commerce/B2B marketplaces are expected to grow fastest over 2025–2034.
Q5 : How is AI affecting the medical disposables market?
A: AI impacts the market across demand forecasting, materials discovery, manufacturing quality control, inventory optimization, regulatory automation, smart disposables/traceability, and clinical decision support, enabling cost savings, faster innovation, and improved outcomes (see AI section above for deep points).
Access our exclusive, data-rich dashboard dedicated to the life sciences sector – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/6086
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Powering Healthcare Leaders with Real-Time Insights: https://www.towardshealthcare.com/healthcare-intelligence-platform
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest