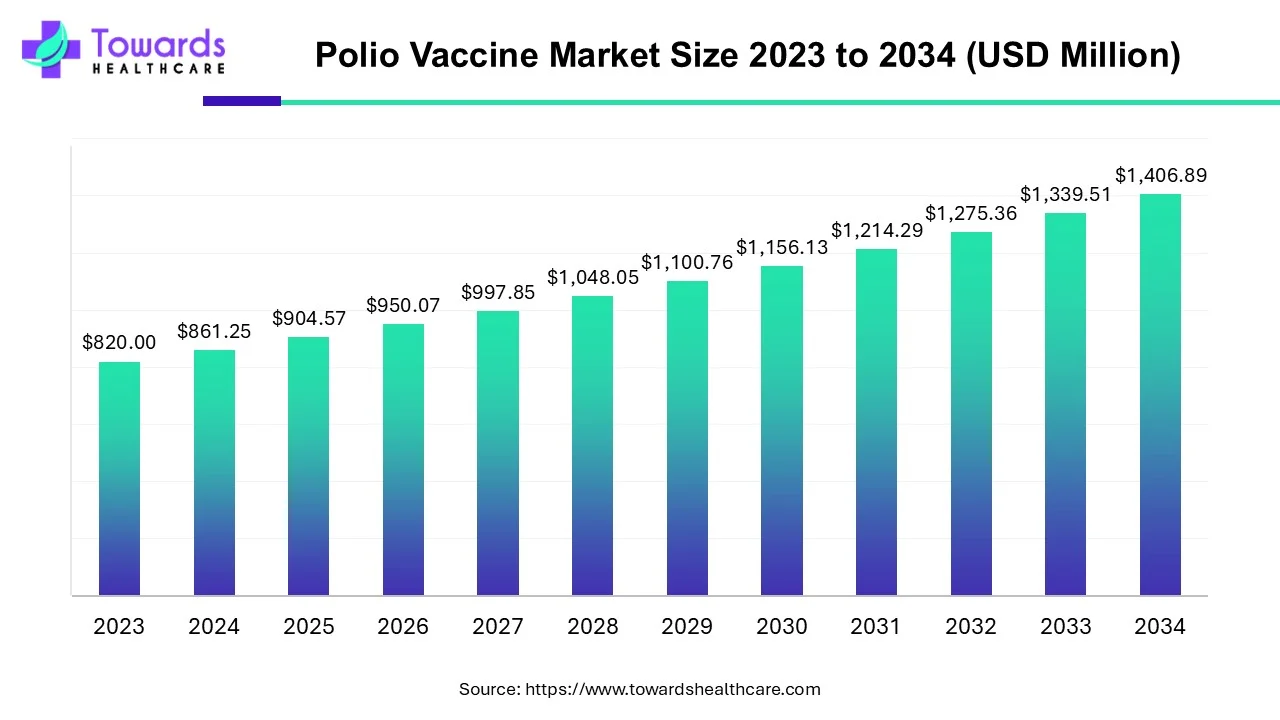
The global polio vaccine market was valued at USD 904.57 million in 2025 and is projected to reach USD 1,406.89 million by 2034, expanding at a CAGR of 5.03%, driven by eradication programs, WHO–UNICEF–GPEI initiatives, technological advancements, and continued outbreaks requiring vaccination.
Download the free sample and get the complete insights and forecasts report on this market @ https://www.towardshealthcare.com/download-sample/5477
Market Size of Polio Vaccine Market
2023 Baseline:
◉Estimated size USD 861.25 million in 2024.
◉Sets the stage for consistent demand due to global eradication commitments.
2025 Market Value:
◉Reaches USD 904.57 million, backed by mass immunization campaigns (e.g., Gaza vaccinating 603,000 children with nOPV2).
2034 Projection:
◉Expected to grow to USD 1,406.89 million, reflecting sustained public-private partnerships and expanded IPV production in Africa and Asia.
Growth Driver:
◉CAGR of 5.03% (2025–2034) indicates steady demand despite polio-free status in several countries, as ongoing outbreak control and travel immunization sustain the market.
Dose Demand Forecasts:
◉UNICEF projects 3.7–3.9 billion bOPV doses needed in 4 years, equating to $500 million expenditure globally.
Over 1 billion nOPV2 doses administered in 35+ countries over the last 3 years.
Market Trends
Eradication Campaigns Fuel Demand:
◉Initiatives like EPI, UIP, NRHM in India and GPEI globally keep vaccines indispensable.
◉In 2024, Guinea immunized 3.2M children via UNICEF campaigns.
Partnerships Boost Local Production:
◉Sanofi + Biovac collaboration (2024) → first IPV manufacturing in Africa.
◉Strengthens supply security and reduces import reliance.
Technology-driven Innovations:
◉Thermostable vaccines & next-gen IPV → minimize cold-chain dependency.
◉China approved msIPV (Vero Cell, Sabin strains) in 2024.
Government Funding Expands Access:
◉Canada pledged $111M to GPEI (2024–2027) → part of its $1B total support.
◉UK contributed £1.65B to GAVI (2021–2025) for global immunization.
Outbreak-Driven Market Activation:
◉74 global polio cases reported (GPEI data).
◉Sudan (2024) → launched emergency campaign against cVDPV2 outbreak.
AI’s Role in Polio Vaccine Market
Supply Chain Optimization
◉AI predicts optimal delivery routes in conflict zones or rural areas.
◉Reduces wastage, ensures cold chain integrity.
Disease Surveillance & Outbreak Prediction
◉ML models detect virus circulation in wastewater (as seen in Germany 2024).
◉AI integrates epidemiological & environmental data for real-time outbreak mapping.
Targeted Vaccination Campaigns
◉AI highlights low-coverage zones → enables rapid “ring vaccination” response.
◉Helps address vaccine hesitancy hotspots with tailored communication.
Clinical Research & Next-Gen Vaccines
◉AI accelerates IPV formulation optimization, mRNA-based vaccine design.
◉Shortens R&D cycle → faster regulatory approvals.
Global Resource Allocation
◉AI systems model dose requirement forecasts (e.g., UNICEF’s 3.7B doses need).
◉Prevents understocking in high-birth regions like India, Pakistan.
Regional Insights in Polio Vaccine Market
1. North America (Market Leader, 2024)
◉Strengths: Strong healthcare infrastructure, stockpiles, CDC surveillance.
◉U.S.: Continues high vaccination coverage despite polio-free status; contributes to GPEI.
◉Canada: Routine immunization + $111M GPEI funding → ensures ongoing demand.
2. Asia-Pacific (Fastest Growth)
◉Drivers: Large child population, rising healthcare spend, active outbreaks.
◉India: Mass campaigns (UIP, NRHM), rural immunization, high birth rates sustain demand.
◉China: NMPA-approved msIPV in 2024; major vaccine producer & consumer.
◉Pakistan & Afghanistan: Endemic polio → continuous OPV campaigns required.
3. Europe (Stable Market)
◉UK: Routine immunization + largest GAVI donor (funding £1.65B).
◉Germany: Detected VDPV2 in wastewater (2024) → ensures vigilance & steady demand.
◉Region-wide: Traveler & migrant vaccination maintains vaccine coverage.
4. Latin America (Rising CAGR)
◉Brazil: Polio-free 34 years, OPV coverage 86.55% in 2023 (up from 77.20%).
◉Mexico: Ongoing “National Public Health Week” drives catch-up vaccinations.
5. Middle East & Africa (Outbreak-Driven Market)
◉Sudan (2024): Emergency vaccination against cVDPV2.
◉Africa (Sanofi–Biovac partnership): First IPV local production → supply resilience.
Market Dynamics
Drivers:
◉Global eradication initiatives (GPEI, WHO, UNICEF).
◉Government funding (e.g., Canada $111M, UK £1.65B).
◉Over 1B nOPV2 doses in 35+ countries in 3 years.
Restraints:
◉Adverse effects (fever, rare VDPV cases).
◉Vaccine hesitancy driven by misinformation.
Opportunities:
◉Next-gen IPV & thermostable vaccines.
◉Needle-free delivery systems.
◉Scaling production in Africa & Asia (Sanofi–Biovac, Sinovac msIPV).
Top Companies in Polio Vaccine Market
Pfizer Inc.
◉Products: Expanding vaccine pipeline (incl. mRNA tech).
◉Strength: Global R&D powerhouse; proven vaccine scalability.
Sanofi
◉Products: IPV portfolio, OPV supply.
◉Strength: Strategic partnerships (Biovac Africa, 2024).
GSK plc
◉Overview: Global immunization leader; vaccines integrated into EPI programs.
◉Strength: Long-standing presence in pediatric vaccines.
Serum Institute of India
◉Products: IPV & OPV bulk production.
◉Strength: Largest vaccine manufacturer; cost-effective supply for LMICs.
BIO-MED & Haffkine Bio-Pharma (India)
◉Products: Local IPV/OPV supply.
◉Strength: Strong government backing in mass immunization campaigns.
Latest announcements
India (2024): National Polio Immunization Drive launched (quote: “Every child under 5 must get drops.”)
Scope & Operational intent
◉Nationally coordinated campaign targeting all children <5 years — implies both routine immunization catch-up and door-to-door or fixed-site activities.
◉Likely to combine mass OPV rounds (for herd immunity) with IPV boosts where available (to reduce VDPV risk).
Resource implications
◉Large vaccine procurement & cold-chain mobilization (syringes for IPV, cold boxes/icepacks for IPV; for OPV oral drops less stringent cold chain but still monitored).
◉Workforce mobilization: vaccinators, community health workers (ASHA/ANM), supervisors, data teams.
Data & monitoring
◉Real-time microplanning, tally sheets, lot-wise dose tracking; emphasis on zero-missed-child strategy.
◉Likely use of line-listings and post-campaign coverage surveys to validate reach.
Public health impact
◉Short term: rapid increase in population immunity among children under 5; reduces susceptible cohort.
◉Medium term: lowers probability of cVDPV emergence where coverage gaps exist.
Policy / signaling
◉Strong political signal of continued vigilance despite India being polio-free; supports domestic and international donor confidence.
◉Reinforces public messaging to counter hesitancy: “every child must get drops” is simple, actionable call-to-action.
Canada (2024): $111M committed to GPEI (bringing total to ~$1B)
Financial mechanics
◉Multi-year funding (noted as $111M over next three years) — provides predictable financing for vaccine procurement, surveillance, outbreak responses.
◉Earmarked funding typically supports vaccine procurement, cold chain, laboratory networks, and campaign logistics in partner countries.
Strategic effects
◉Strengthens GPEI operational capacity (surge staffing, emergency stockpiles, surveillance upgrades).
◉Encourages co-funding from other donors; can be leveraged to secure multi-national initiatives (e.g., regional response pools).
Market & supply effects
◉Stable donor funding reduces procurement volatility; manufacturers can plan production volumes (important for nOPV2 and bOPV demand forecasts).
Reputational / diplomatic
◉Positions Canada as a sustained global health donor — helps in negotiating procurement frameworks and advocating global procurement standards.
Recent developments
Sudan (Nov 2024): Emergency campaign vs cVDPV2
Situation: cVDPV2 detection triggered emergency mass vaccination for children <5.
Operational response details
◉Rapid microplanning, mop-up rounds, targeted high-risk geographies.
◉Likely use of nOPV2 or mOPV2 in line with international guidance for cVDPV2 outbreaks.
Epidemiologic implication
◉Emergency campaigns reduce viral circulation quickly if high coverage achieved; failure to reach pockets risks continued transmission and further genetic drift.
Market implication
◉Increased short-term demand for OPV variants (nOPV2/mOPV2), logistical procurement, surge staffing.
China (Apr 2024): Sinovac’s msIPV (Vero Cell, Sabin strains) approved by NMPA
Product specifics
◉msIPV: Sabin-strain based, Vero cell manufacturing platform, five-dose presentation (implies multi-dose vial).
Regulatory & production impact
◉Domestic approval supports China’s self-sufficiency and export potential to markets accepting Sabin-strain IPV.
◉Multi-dose vials may reduce per-dose cost but increase requirements for safe vial handling and open-vial policies.
Programmatic effect
◉Availability of msIPV can support routine immunization and catch-up campaigns where IPV is preferred to eliminate VDPV risk.
Market dynamics
◉Adds supply diversity, potentially lowers prices and increases competition for IPV market share (especially in Asia/Africa where price sensitivity matters).
Guinea (2024): 3.2 million children immunized via UNICEF partnership
Scale & delivery
◉Two-round initiative indicates coordinated campaign strategy — coverage aims to interrupt transmission/clusters.
Operational learning
◉Demonstrates effective partnership model (government + UNICEF + partners) for rapid large-scale vaccination in resource-limited settings.
Epidemiologic signal
◉High coverage rounds are effective at rapidly increasing herd immunity in outbreak or high-risk settings.
Market note
◉Reinforces OPV’s role for mass campaigns given ease of administration.
Gaza (Feb 2025): ~603,000 children immunized with nOPV2 during ceasefire
Contextual notes
◉Use of nOPV2 (novel OPV type 2) aimed at outbreak control with reduced reversion risk vs traditional OPV2.
◉The added 40,000 children vs prior rounds suggests improved access during the ceasefire window.
Operational challenges & achievements
◉Vaccination in conflict settings requires negotiation for humanitarian access, secure cold chain corridors, and mobile teams.
◉Successful campaign indicates operational resilience and ability to vaccinate in fragile settings.
Market impact
◉Demand for nOPV2 increases; donors and WHO need to ensure sustained supply and buffer stockpiles.
Segments covered
By Type — deep technical & programmatic contrasts
1. Inactivated Polio Vaccine (IPV)
Biological/technical features
◉Composition: Killed poliovirus (Salk/IPV) or Sabin-strain inactivated (msIPV). Cannot replicate — no VDPV risk.
◉Delivery: Intramuscular or subcutaneous injection; requires trained personnel and injection safety (syringes, sharps disposal).
Programmatic role
◉Routine immunization backbone in polio-free or high-income settings to maintain individual protection.
◉Used in combination schedules where OPV is used for mass campaigns but IPV ensures individual humoral immunity without VDPV risk.
Advantages
◉No vaccine-derived poliovirus; safer for endemically polio-free populations.
◉Acceptable in settings with high vaccine hesitancy about live vaccines.
Constraints
◉Higher per-dose cost than OPV; injection logistics (cold chain, sterile devices).
◉Requires higher cold-chain reliability for multi-dose vials; training for injection safety.
Market & manufacturing
◉Growth driven by partnerships (e.g., Sanofi–Biovac) to localize production — increases supply, reduces lead times.
msIPV approvals (e.g., Sinovac) increase competitive supply and price pressure.
2. Oral Polio Vaccine (OPV)
Biological/technical features
◉Composition: Live attenuated poliovirus (Sabin strains) administered orally.
◉Delivery: Oral drops — simple, no needles, ideal for mass campaigns and door-to-door rounds.
Programmatic role
◉Primary tool for mass immunization & outbreak control due to ease of administration and induction of intestinal immunity (interrupts transmission).
Advantages
◉Low cost, easy to administer en masse (volunteers, minimally trained staff).
◉Induces mucosal immunity — better at stopping community spread than IPV alone.
Constraints & risks
◉Rare risk of Vaccine-Derived Poliovirus (VDPV), especially in settings with low coverage; drives development of nOPV2 (reduced reversion risk).
◉Requires high coverage to avoid paradoxical emergence of VDPV.
Market & operational
◉Because of mass campaign utility, OPV demand surges during outbreaks (e.g., Guinea, Gaza, Sudan).
◉UNICEF’s procurement forecasts (3.7–3.9B bOPV doses over 4 years) highlight scale and budgetary planning needs.
By End-user
Hospitals & Clinics (dominant channel)
Role & workflow
◉Primary point for routine IPV immunization and catch-up injections; equipped for injection safety, clinical monitoring for AEFI (adverse events following immunization).
◉Central to cold-chain maintenance for injectable vaccines.
Economics
◉Higher per-dose handling costs (staff, consumables) but provides reliable coverage and clinical oversight.
Quality & surveillance
◉Frontline for AEFI surveillance and reporting; labs for confirmatory testing may be associated.
Public Services (growing, outreach focus)
Role & workflow
◉Government-led campaigns: mass OPV rounds, school-based drives, community outreach.
◉Mobilizes nonclinical workforce, volunteers, and mobile outreach units.
Programmatic importance
◉Essential to reach remote, underserved populations — reduces inequity in immunization.
◉Often funded by national budgets plus donor support (GPEI, UNICEF, GAVI).
Operational tradeoffs
◉Campaigns deliver high coverage quickly but need meticulous microplanning to avoid missed pockets that can seed VDPV.
By Region
North America
Public health context
◉Polio-free status; focus on maintaining high IPV coverage, stockpiles, and rapid outbreak readiness.
Market features
◉Higher price tolerance; procurement emphasizes quality, regulatory compliance (FDA/Health Canada).
Surveillance & R&D
◉Strong lab networks (wastewater, AFP surveillance) and contribution to global funding (e.g., Canada $111M).
Asia-Pacific
Diverse epidemiology
◉Countries range from polio-free to high-risk (Pakistan/Afghanistan). India is polio-free but maintains mass immunization capability.
Market dynamics
◉Large absolute dose demand due to population & birth cohort size — significant for UNICEF forecasts.
Manufacturing & supply
◉Growing local manufacturing (Sinovac msIPV, Serum Institute production) reduces regional dependency.
Operational complexity
◉Rural access, cold chain upgrades, and addressing vaccine hesitancy are major program costs.
Europe
Surveillance emphasis
◉Wastewater surveillance detected VDPV2 signals (noted in 14 cities across five countries in 2024) — drives monitoring programs.
Policy stance
◉Maintains routine IPV schedules and readiness for targeted OPV use if importation occurs.
Travel & migration
◉Vaccination of travelers and migrants from endemic areas is policy nuance that sustains demand.
Latin America
Campaign history
◉Longstanding OPV campaigns; historical success (Brazil polio-free 34 years) but coverage fluctuations require catch-ups.
Operational drivers
◉National public health weeks and free vaccine drives boost coverage; public-private participation common.
Middle East & Africa (MEA)
Fragility & outbreaks
◉Conflict zones (Gaza), fragile states (Sudan) increase outbreak risk and complicate campaign delivery.
Manufacturing opportunity
◉Sanofi–Biovac IPV production in Africa supports supply resilience and regional self-reliance.
Donor dependency
◉Heavy reliance on GPEI/UNICEF funding for campaign implementation and vaccine procurement.
Top 5 FAQs
-
What is the projected size of the polio vaccine market by 2034?
→ USD 1,406.89 million, growing at a CAGR of 5.03%. -
Which vaccine type is dominant in the market?
→ IPV dominates due to safety, while OPV is the fastest-growing for mass campaigns. -
Which regions drive growth?
→ North America leads in 2024; Asia-Pacific grows fastest due to population and outbreaks. -
How many doses are needed globally?
→ UNICEF estimates 3.7–3.9B bOPV doses in 4 years, worth $500M. -
What are the recent major developments?
→ Sudan’s 2024 cVDPV2 campaign, Sinovac’s msIPV approval in China, and Gaza’s 2025 nOPV2 drive.
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5477
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Powering Healthcare Leaders with Real-Time Insights: https://www.towardshealthcare.com/healthcare-intelligence-platform
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest