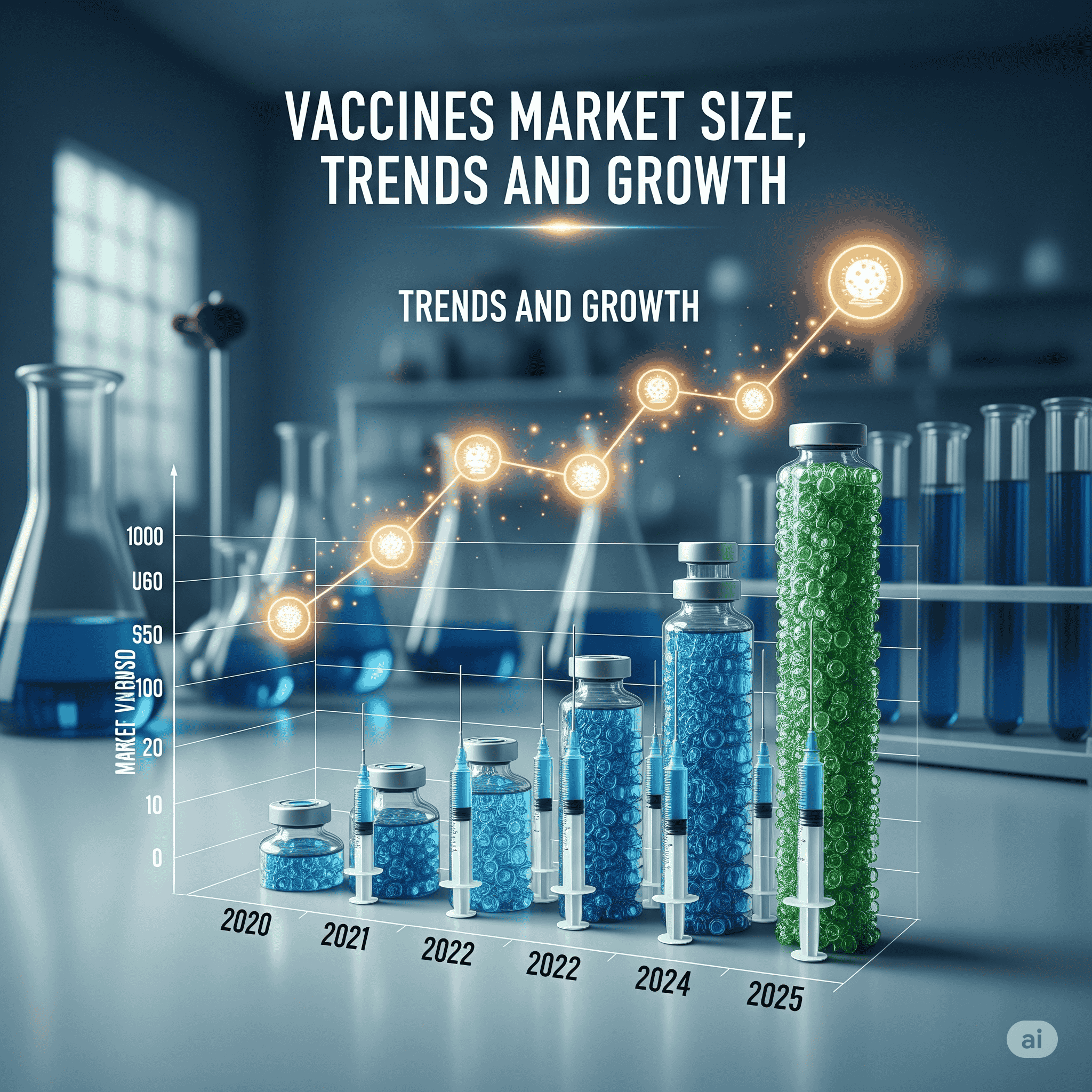
The global vaccines market was valued at USD 81.67 billion in 2024, expanded to USD 84.97 billion in 2025, and is projected to reach USD 121.36 billion by 2034, registering a CAGR of 4.04% during 2025–2034. Growth is driven by government immunization programs, AI-enabled vaccine R&D, rising infectious diseases, and strong collaborations between public and private organizations. North America dominated in 2024, while Asia-Pacific is projected to be the fastest-growing region.

Download the free sample and get the complete insights and forecasts report on this market @ https://www.towardshealthcare.com/download-sample/6008
Market Size
◉2024 – USD 81.67 billion (baseline year, dominated by recombinant/subunit vaccines and pediatric immunizations).
◉2025 – USD 84.97 billion, reflecting stable growth, supported by government tenders and procurement.
◉2030 – Market expected to cross USD 105 billion, driven by nucleic-acid platforms (mRNA/DNA vaccines) and seasonal respiratory vaccines.
◉2034 – USD 121.36 billion, with increasing role of contract manufacturing (CDMOs) and advance purchase agreements (APAs).
◉CAGR (2025–2034) – 4.04%, moderate but steady, reflecting maturity of traditional vaccines alongside rapid adoption of AI-driven genomic technologies.
Market Trends
Vaccination Drives
◉August 2025: Tamil Nadu, India conducted a Japanese Encephalitis drive in schools & anganwadis.
◉Shows rising regional and local vaccination campaigns to prevent vector-borne outbreaks.
New Product Launches
◉March 2025: Malaria vaccine (RTS,S) introduced in Burundi by Gavi, UNICEF, WHO.
◉Marks integration of malaria vaccines into routine immunization programs.
Shift Towards Next-Gen Vaccines
◉mRNA and DNA vaccines showing fastest growth.
◉Easy adaptability to multiple strains & pathogens makes them critical for pandemic preparedness.
Therapeutic Vaccines Development
◉Cancer vaccine R&D, especially against KRAS-mutated pancreatic cancers (Phase 1 success in Aug 2025).
◉Expands vaccines beyond infectious diseases into oncology applications.
Government Procurement Dominance
◉2024: Government tender & bulk procurement dominated distribution models.
◉Ensures accessibility in low & middle-income countries.
Advance Purchase Agreements (APAs)
◉Fastest-growing segment – APAs de-risk investment and accelerate R&D timelines.
Role of Artificial Intelligence in the Vaccines Market
Vaccine Design & Development
◉AI predicts antigenic epitopes and identifies immune response triggers.
◉Example: AI models screen vast genomic datasets to find ideal vaccine targets.
Acceleration of R&D Timelines
◉Reduces development cycle from 10–15 years to 2–5 years (as seen in COVID-19 mRNA vaccines).
Real-Time Monitoring & Prediction
◉AI-enabled clinical trial monitoring improves safety tracking.
◉Predicts vaccine outcomes and long-term population immunity dynamics.
Improved Delivery & Stability
◉ML models optimize formulation and cold-chain requirements, lowering logistical barriers.
Adaptive Platforms for Emerging Diseases
◉AI-driven platforms can rapidly adapt to mutating pathogens like influenza or SARS-CoV-2.
Personalized Vaccine Approaches
◉AI enables neoantigen-based therapeutic vaccines (e.g., cancer immunotherapy).
Regional Insights
North America (Dominant in 2024)
◉Factors: Strong healthcare infrastructure, R&D leadership, government programs.
U.S.:
◉Hosts 4,207 vaccine trials (out of 10,771 worldwide, Aug 2025).
◉Major vaccine manufacturers: Pfizer, Moderna, GSK.
Canada:
◉Invested $317M in CanGIVE initiative to boost vaccine equity & manufacturing.
Asia-Pacific (Fastest Growing)
◉Drivers: High infectious disease burden, large population, growing collaborations.
China:
◉2024–25 flu season dominated by H1N1 (97.4%).
◉Low influenza vaccination coverage (2.46%).
India:
◉UIP provides free vaccines to 2.9 crore pregnant women & 2.6 crore infants annually.
◉U-WIN platform digitalizes immunization tracking.
Europe
◉Growth Factors: Favorable policies, advanced research infrastructure.
Belgium:
◉Largest vaccine exporter ($15.3B, 2023).
Ireland:
◉2nd largest exporter ($12.3B, 2023).
◉Vaccines = 3rd most exported product.
Latin America
◉Brazil & Mexico: Increasing adoption of routine immunization + private sector entry.
Middle East & Africa (MEA)
◉UAE, Saudi Arabia, South Africa investing in vaccine storage & manufacturing hubs.
◉Focus on infectious disease prevention (e.g., Ebola, malaria, dengue).
Recent developments
Clinical / R&D milestones
◉KRAS-targeting pancreatic cancer vaccine (Phase 1 positive, Aug 2025). Demonstrates the growing frontiers of therapeutic/oncology vaccines beyond infectious disease.
◉Multi-virus vaccine pipeline (La Jolla Institute, Aug 2025) — design approaches aimed at broad neutralization across SARS-CoV-2 variants, illustrating cross-pathogen antigen design.
Public health rollouts & campaigns
◉Malaria vaccine integration (Burundi, Mar 2025) — RTS,S/other malaria vaccine doses rolled into routine immunization schedules via Gavi/UNICEF/WHO collaboration.
◉Regional vaccination drives (Tamil Nadu JE drive, Aug 2025) — local mass immunization efforts targeting vector-borne disease in schools and anganwadis.
Market / policy actions
◉APAs & pre-buy mechanisms increasingly used post-COVID to de-risk developer investments and secure supply for governments and global initiatives. (See Advance Market Commitment concept.)
Segments covered
By vaccine technology / platform
Subunit / conjugate / recombinant protein vaccines
◉Widely adopted for pediatric routine immunizations and many established vaccines; dominate revenue share in 2024 due to manufacturing scale, safety profile, and broad portfolio presence.
◉mRNA & other nucleic-acid vaccines (mRNA/DNA)
◉Fastest growth projected — rapid design/scale, adaptable to variants; require LNP formulation and cold-chain considerations.
Viral-vector vaccines (replicating & non-replicating)
◉Useful for single-dose strategies and certain outbreak responses.
Live attenuated & inactivated/killed vaccines
◉Longstanding platforms with mature manufacturing but slower adaptability than nucleic-acid platforms.
VLPs & peptide vaccines / therapeutic vaccines
◉Rising in oncology and chronic infections; peptide neoantigen vaccines exemplify precision personalization.
By product & manufacturing stage
◉Finished vaccine products (vials, prefilled syringes) — largest revenue share due to ease of administration and established cold-chain logistics.
◉Fill-finish & packaging — critical bottleneck and margin center; surge in CDMO demand.
◉Adjuvants & formulation components — specialized inputs enabling dose-sparring and improved immunogenicity.
◉Commercial scale manufacturing vs. contract manufacturing (CDMOs)
◉Commercial scale dominates revenue (large producers).
◉Contract manufacturing fastest growing — biotech startups rely on CDMOs for GMP antigen, LNP, and fill/finish.
By distribution / commercial model
◉Government tender & bulk procurement — primary route for national immunization programs; stabilizes large volume demand.
◉Advance Purchase Agreements (APAs), AMCs — used to secure early supply and fund R&D (COVAX/COVAX-AMC precedent).
◉Private/commercial & B2B (CDMO) channels — supplements public programs and provides market for new product launches.
By region (structural)
◉North America — R&D, high unit prices, clinical trial concentration.
◉Asia-Pacific — volume growth, manufacturing scale (e.g., SII), fastest CAGR projection.
◉Europe / LATAM / MEA — mix of export hubs (Belgium/Ireland), regional manufacturing investments, expanding public procurement.
Top companies (deep, point-wise: product, overview, core strength)

Pfizer / BioNTech
Key products: Comirnaty (mRNA COVID-19), multiple mRNA development programs.
Overview: Global leader in distribution networks; large commercial footprint and regulatory experience.
Strength: mRNA commercialization, cold-chain logistics, global government contracts.
Moderna
Key products: mRNA-1273 (COVID platform) + pipeline (respiratory, oncology).
Overview: Pure-play mRNA platform company.
Strength: Rapid design/manufacturing agility and LNP expertise. Wikipedia
GlaxoSmithKline (GSK)
Key products: Wide portfolio (e.g., shingles, RSV candidates, adjuvants).
Overview: Large legacy vaccine portfolio and adjuvant R&D.
Strength: Vaccine R&D breadth and public-programme partnerships.
Sanofi Pasteur
Key products: Established childhood and adult vaccines; strategic partnerships for mRNA/novel tech.
Strength: Global immunization program penetration and manufacturing scale.
Merck & Co. (MSD)
Key products: Gardasil (HPV), other routine vaccines.
Strength: Strong prophylactic vaccine brands and long-term programmatic ties.
Serum Institute of India (SII)
Key products: High-volume producers of multiple routine vaccines and COVID doses.
Strength: Global largest by dose volume, cost-efficient manufacturing for LMIC supply.
Novavax
Key products: Protein subunit COVID & influenza candidates.
Strength: Subunit platform expertise (complementary to mRNA).
Sinovac / Sinopharm / Bharat Biotech / CSL Seqirus / Valneva / Bavarian Nordic / Emergent
Overview & strength: Regional market access, specialized platforms (inactivated, viral vector, cryo-stable formulations), and manufacturing capacity for public procurements.
Market dynamics
Primary drivers
◉Government immunization programs & procurement — large, predictable demand; public financing reduces commercial risk.
◉Unmet disease burden & seasonal respiratory infections — drives recurrent vaccine demand and booster strategies.
◉Technological platforms (mRNA, VLPs) — open new product classes and accelerate candidate throughput.
Key restraints
◉Adverse event concerns & vaccine hesitancy — can reduce uptake and political willingness to fund programs.
◉Cold-chain and distribution constraints — especially for fragile mRNA products in LMICs.
◉Consolidation & market concentration — economic barriers for new entrants, risk of monopolistic pricing.
Enabling mechanisms / opportunities
◉APAs / AMCs — assure demand and fund R&D; proven in pneumococcal and COVID responses.
◉CDMO expansion — allows biotech innovators to scale without heavy capital investment.
◉AI & genomic tech — shortens antigen selection cycles and supports precision therapeutic vaccines.
Government healthcare spending & policies
1 Public financing mechanisms & procurement models
Direct budgetary appropriations to national immunization programs
◉Governments allocate recurring health budgets to childhood & adult vaccination programs; spending typically covers procurement (tenders), cold-chain logistics, human resources, and surveillance. Public tenders dominate the distribution model (largest 2024 share in your data).
Advance Purchase Agreements (APAs) / Advance Market Commitments (AMCs)
◉Mechanism: Governments (or coalitions) commit to purchase volumes at pre-agreed prices if developer meets criteria, thus derisking private investment and enabling surge capacity. Proven in pneumococcal and COVID vaccine responses.
Multilateral financing & donor programs (Gavi, UNICEF, WHO)
◉Low-/middle-income country support via subsidized procurement, tiered pricing, and donation channels; can accelerate rollout (example: Rwanda/Gavi models historically, and Burundi malaria rollout in 2025 in your text).
2 Policy levers that shape market incentives
Regulatory frameworks & accelerated approval pathways
◉Emergency Use Authorizations and adaptive licensing accelerate availability in outbreaks, but also shift long-term post-market surveillance obligations.
Reimbursement & coverage policies
◉National vaccine schedules and public funding (e.g., free childhood vaccines) determine demand volume and manufacturer strategy (e.g., Canada’s CanGIVE investment in manufacturing equity).
Export/industrial policy & trade incentives
◉Countries with strong pharma export policies (Belgium, Ireland) attract manufacturing investment; tax incentives/grants are used to build vaccine production hubs.
3 Spending trends & priorities (programmatic emphasis)
Pandemic preparedness & surge capacity
◉Post-COVID, many governments maintain funding lines for strategic vaccine stockpiles, secured via APAs and pre-buy mechanisms.
Equity & access spending
◉Programs like CanGIVE and Gavi-led initiatives target vaccine equity, funding cold-chain expansion and subsidized doses for pregnant women/infants (e.g., India’s UIP statistics you provided).
R&D and platform investments
◉Public funding often targeted at platform tech (mRNA, adjuvants) and translational pipeline grants — signals to private sector via co-funding and contracts.
4 Policy risks & political drivers
Risk of political shifts impacting funding (example signals in 2025 news)
◉Political leadership changes can alter R&D funding priorities, procurement preferences (platform bias), and regulatory stances; sudden policy reversals can create market uncertainty. (See vaccination policy and national policy frameworks.)
Vaccine hesitancy & mandates
◉Policies on mandates, school entry requirements, and travel vaccination rules influence uptake; governments must balance mandates with public sentiment and legal frameworks.
Technological disruption & innovations
1 Platform innovations
◉mRNA & nucleic acid platforms
◉Rapid design (days to candidate), standardized manufacturing runs, and strong scalability make mRNA a central disruptive technology; trade-offs include LNP formulation complexity and cold-chain demands.
Viral-vector & VLP engineering
◉Offer single-dose efficacy for some indications; antigen display tech allows multi-epitope vaccines.
2 AI / computational biology
Epitope prediction & antigen selection
◉ML models screen large genomic/proteomic datasets to prioritize antigen candidates, reducing preclinical attrition.
In silico trial design & predictive safety
◉Simulations reduce trial sizes and improve dose/regimen selection.
Formulation & stability optimization
◉AI models optimize excipient mixes and LNP compositions to enhance thermostability (critical for distribution in LMICs).
3 Manufacturing & supply chain innovations
Modular & decentralized manufacturing
◉Smaller, modular mRNA or fill–finish plants allow regional scale-up and reduce reliance on centralized hubs.
Continuous manufacturing & automation
◉Reduce per-dose costs, increase batch consistency, and cut lead times.
Cold-chain tech & thermostable formulations
◉Innovations in lyophilization, improved LNPs, and ambient-stable formulations lessen distribution bottlenecks.
4 Clinical & regulatory innovations
Adaptive trial designs & platform trials
◉Platform trials allow parallel testing of multiple vaccine candidates, accelerating go/no-go decisions.
Real-world evidence & digital pharmacovigilance
◉Post-market safety monitoring using digital records and AI anomaly detection improves public confidence and regulatory responsiveness.
5 Product innovations & new indications
Therapeutic vaccines (oncology)
◉Neoantigen vaccines and peptide-based off-the-shelf cancer vaccines redefine the market beyond prophylaxis.
Multi-pathogen & universal vaccines
◉Designs aimed at pan-coronavirus or universal influenza targets reduce need for yearly reformulation and represent major disruption if realized.
Short synthesis & implications
◉Government policy & spending continue to be the primary demand engine (tenders, APAs, immunization budgets) — manufacturers must align capacity with procurement cycles and policy incentives.
◉mRNA & AI are structural disruptors — they shrink R&D timelines and enable personalized therapeutics but require policy support (funding, regulatory clarity) and investments in manufacturing/cold-chain.
◉Equity mechanisms (Gavi, CanGIVE, AMCs) will remain crucial to ensure global access and to stabilize demand forecasts for manufacturers
Top 5 FAQs
Q1: What is the current and projected market size of the vaccines market?
USD 81.67B (2024) → USD 121.36B (2034) at 4.04% CAGR.
Q2: Which region dominated the vaccines market in 2024?
North America, due to robust R&D and presence of Pfizer, Moderna, GSK.
Q3: Which region will grow fastest during 2025–2034?
Asia-Pacific, fueled by rising disease burden, population, and government immunization drives.
Q4: Which vaccine types are leading and fastest growing?
Leading: Subunit/conjugate/recombinant protein vaccines.
Fastest growing: mRNA & nucleic acid vaccines.
Q5: What are the major opportunities in the future vaccines market?
AI-powered R&D, therapeutic cancer vaccines, genomic technology-driven discovery, and APA-backed pandemic preparedness.
Access our exclusive, data-rich dashboard dedicated to the therapeutic area sector – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/6008
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region – +44 778 256 0738
North America Region – +1 8044 4193 44
Web: https://www.towardshealthcare.com
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest