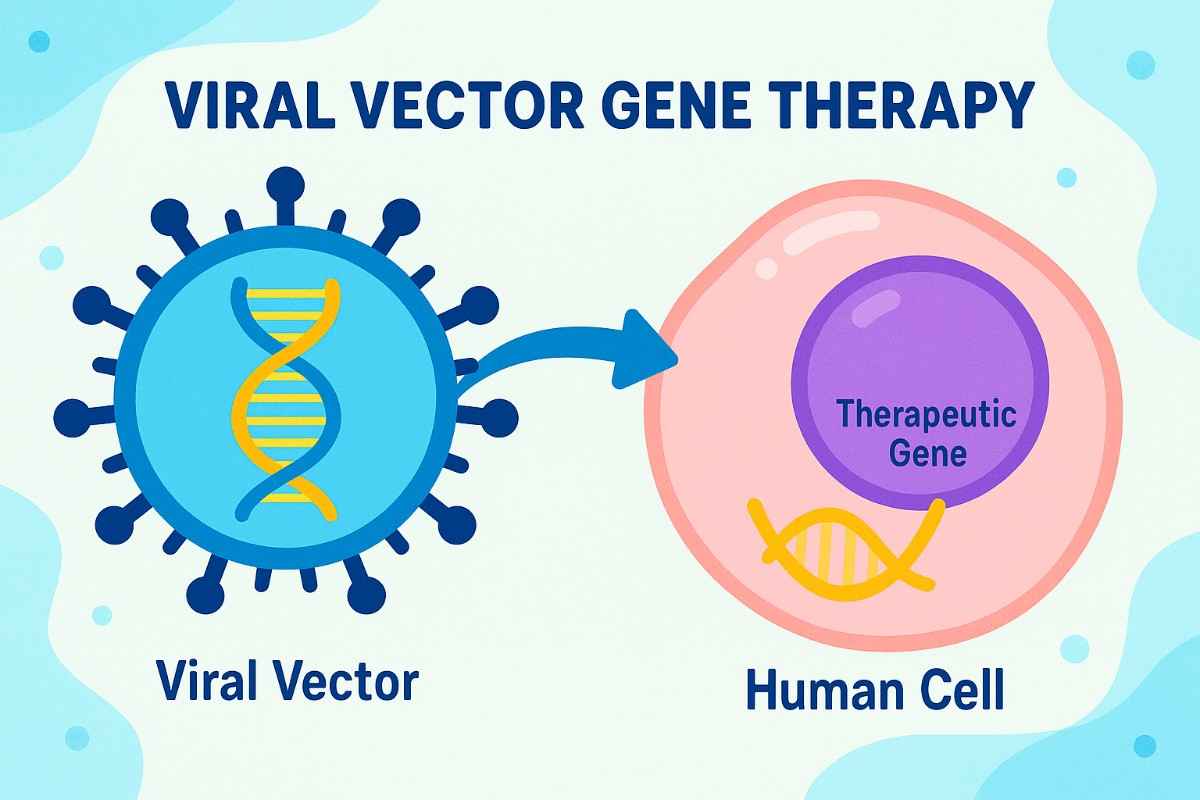
The global viral vector gene therapy market is entering a transformative era, witnessing rapid expansion and growing relevance across healthcare sectors. From tackling genetic disorders to enabling cutting-edge personalized medicine, this field is redefining how we treat some of the world’s most challenging diseases.
Why Is the Market Growing So Rapidly
The market’s momentum stems from a combination of major advancements:
-
Advancing genetic engineering technologies
-
Rising demand for targeted, long-lasting treatments
-
Regulatory support and increased research and development investments
These forces are enabling pharmaceutical companies, CROs, and CDMOs to push forward with promising clinical trials and therapeutic breakthroughs.
Invest in Our Premium Strategic Solution: https://www.towardshealthcare.com/download-databook/5737
Key Market Insights in 2024
-
North America led the market in 2024, driven by advanced healthcare infrastructure and high research activity
-
Asia-Pacific is projected to grow at the fastest rate through 2034, due to rising investment and healthcare accessibility
-
Adeno-associated virus vectors dominated the market due to strong safety and clinical effectiveness
-
Lentivirus vectors are expected to see the highest growth, offering durable gene expression capabilities
-
Gene therapy applications held the largest revenue share and will continue leading market expansion
-
Pharmaceutical and biotechnology firms are the primary users of viral vector gene therapy
-
CROs and CMOs are rapidly expanding in usage, reflecting increased outsourcing trends
What Is Viral Vector Gene Therapy
Viral vector gene therapy uses engineered viruses to deliver healthy genes into patient cells. These vectors are designed to correct or replace faulty genes, offering highly targeted and potentially curative treatments for genetic, rare, and chronic diseases.
Advancements in vector design and delivery systems have significantly improved the safety and effectiveness of these therapies.
Get All the Details in Our Solutions – Access Report Preview: https://www.towardshealthcare.com/download-sample/5737
Key Developments in 2025 Shaping the Market
Artificial Intelligence Accelerating Growth
Artificial Intelligence is transforming gene therapy by
-
Identifying optimal gene targets and vector designs
-
Enhancing the efficiency of clinical trial development
-
Improving manufacturing processes
-
Reducing overall development costs and time
This integration of AI is expected to drive faster commercialization of gene therapies.
Strong Pipeline and Clinical Success
In April 2025, Vertex Pharmaceuticals reported positive Phase 3 results for its CRISPR-Cas9-based therapy for sickle cell disease, significantly reducing vaso-occlusive crises.
In April 2024, Pfizer’s Beqvez received FDA approval for hemophilia B. This AAV-based gene therapy reduces the need for frequent clotting factor infusions, showcasing the power of gene correction strategies.
If you have any questions, please feel free to contact us at sales@towardshealthcare.com
Market Drivers and Restraints
Market Driver – Rising Prevalence of Genetic and Rare Disorders
As diagnostic tools improve, more patients are being identified with genetic diseases that may be treatable through gene therapy. These therapies offer the potential for long-lasting results by addressing the root cause of disease.
Example – In November 2024, the FDA approved Kebilidi, an AAV-based gene therapy for AADC deficiency. The treatment, delivered directly into the brain, enables dopamine production for patients with this rare condition.
Market Restraint – High Cost and Manufacturing Complexity
Viral vector manufacturing remains highly complex and expensive. It requires specialized equipment, skilled personnel, and strict regulatory compliance. These challenges make scaling production difficult and keep treatment costs high, limiting access and affordability.
Segmental Insights
Vector Types Leading the Market
-
Adeno-Associated Virus AAV vectors lead the market due to their low toxicity and targeted delivery capability
-
Lentivirus vectors are gaining popularity for their ability to integrate genetic material into dividing and non-dividing cells, ideal for long-term gene expression
In 2024, NewBiologix launched the Xcell Platform, designed to enhance viral vector production efficiency. Built on a proprietary cell line, the system supports the screening of multiple rAAV candidates and streamlines data collection for regulatory compliance.
Application and End-User Analysis
-
Gene therapy dominates the application segment, driven by advancements in treating rare and chronic conditions
-
Pharmaceutical and biotechnology companies remain the primary users of viral vector-based therapies
-
Contract Research Organizations and Contract Manufacturing Organizations are growing rapidly as outsourcing becomes more prevalent
Industry Milestones and Collaborations
-
In February 2025, Novartis partnered with a major manufacturer to scale up AAV production and improve manufacturing reliability
-
In July 2024, Abeona Therapeutics collaborated with Beacon Therapeutics to develop AAV204-based therapies for eye diseases
-
In May 2023, Regenxbio presented its findings on AAV vector distribution and targeting efficiency at the ASGCT Annual Meeting
Conclusion
With clinical breakthroughs, AI-driven innovations, and increasing investment, the viral vector gene therapy market is positioned for robust growth between 2025 and 2034. As technological and regulatory barriers are overcome, gene therapies will become more accessible, affordable, and impactful across a broader range of diseases.
Source : https://www.towardshealthcare.com/insights/viral-vector-gene-therapy-market-sizing